β-Estradiol 17-acetate enhances the in vitro vitality of endothelial cells isolated from the brain of patients subjected to neurosurgery
- PMID: 35900435
- PMCID: PMC9396507
- DOI: 10.4103/1673-5374.346054
β-Estradiol 17-acetate enhances the in vitro vitality of endothelial cells isolated from the brain of patients subjected to neurosurgery
Abstract
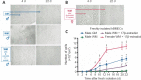
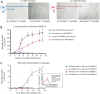
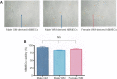
In the current landscape of endothelial cell isolation for building in vitro models of the blood-brain barrier, our work moves towards reproducing the features of the neurovascular unit to achieve glial compliance through an innovative biomimetic coating technology for brain chronic implants. We hypothesized that the autologous origin of human brain microvascular endothelial cells (hBMECs) is the first requirement for the suitable coating to prevent the glial inflammatory response triggered by foreign neuroprosthetics. Therefore, this study established a new procedure to preserve the in vitro viability of hBMECs isolated from gray and white matter specimens taken from neurosurgery patients. Culturing adult hBMECs is generally considered a challenging task due to the difficult survival ex vivo and progressive reduction in proliferation of these cells. The addition of 10 nM β-estradiol 17-acetate to the hBMEC culture medium was found to be an essential and discriminating factor promoting adhesion and proliferation both after isolation and thawing, supporting the well-known protective role played by estrogens on microvessels. In particular, β-estradiol 17-acetate was critical for both freshly isolated and thawed female-derived hBMECs, while it was not necessary for freshly isolated male-derived hBMECs; however, it did counteract the decay in the viability of the latter after thawing. The tumor-free hBMECs were thus cultured for up to 2 months and their growth efficiency was assessed before and after two periods of cryopreservation. Despite the thermal stress, the hBMECs remained viable and suitable for re-freezing and storage for several months. This approach increasing in vitro viability of hBMECs opens new perspectives for the use of cryopreserved autologous hBMECs as biomimetic therapeutic tools, offering the potential to avoid additional surgical sampling for each patient.
Keywords: 17β-estradiol; cryopreservation; gender-specific; gray matter; human brain microvascular endothelial cells; surgical resections; vascular protection; white matter; β-estradiol 17-acetate.
Conflict of interest statement
None
Figures
References
-
- Alemany M. Estrogens and the regulation of glucose metabolism. World J Diabetes. 2021;12:1622–1654. Goncharov NV, Popova PI, Avdonin PP, Kudryavtsev IV, Serebryakova MK, Korf EA, Avdonin PV (2020) Markers of endothelial cells in normal and pathological conditions. Biochem (Mosc) Suppl Ser A Membr Cell Biol 14:167-183. - PMC - PubMed
-
- Alvarez RJ, Gips SJ, Moldovan N, Wilhide CC, Milliken EE, Hoang AT, Hruban RH, Silverman HS, Dang CV, Goldschmidt-Clermont PJ. 17beta-estradiol inhibits apoptosis of endothelial cells. Biochem Biophys Res Commun. 1997;237:372–381. - PubMed
-
- Bordenave L, Fernandez P, Rémy-Zolghadri M, Villars S, Daculsi R, Midy D. In vitro endothelialized ePTFE prostheses:clinical update 20 years after the first realization. Clin Hemorheol Microcirc. 2005;33:227–234. - PubMed
-
- Bouafsoun A, Helali S, Othmane A, Kerkeni A, Prigent AF, Jaffrézic-Renault N, Bessueille F, Léonard D, Ponsonnet L. Evaluation of endothelial cell adhesion onto different protein/gold electrodes by EIS. Macromol Biosci. 2007;7:599–610. - PubMed
LinkOut - more resources
Full Text Sources

