Characteristic fixation biases in Super-Recognizers
- PMID: 35900724
- PMCID: PMC9344214
- DOI: 10.1167/jov.22.8.17
Characteristic fixation biases in Super-Recognizers
Abstract
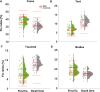
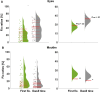
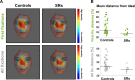
Neurotypical observers show large and reliable individual differences in gaze behavior along several semantic object dimensions. Individual gaze behavior toward faces has been linked to face identity processing, including that of neurotypical observers. Here, we investigated potential gaze biases in Super-Recognizers (SRs), individuals with exceptional face identity processing skills. Ten SRs, identified with a novel conservative diagnostic framework, and 43 controls freely viewed 700 complex scenes depicting more than 5000 objects. First, we tested whether SRs and controls differ in fixation biases along four semantic dimensions: faces, text, objects being touched, and bodies. Second, we tested potential group differences in fixation biases toward eyes and mouths. Finally, we tested whether SRs fixate closer to the theoretical optimal fixation point for face identification. SRs showed a stronger gaze bias toward faces and away from text and touched objects, starting from the first fixation onward. Further, SRs spent a significantly smaller proportion of first fixations and dwell time toward faces on mouths but did not differ in dwell time or first fixations devoted to eyes. Face fixation of SRs also fell significantly closer to the theoretical optimal fixation point for identification, just below the eyes. Our findings suggest that reliable superiority for face identity processing is accompanied by early fixation biases toward faces and preferred saccadic landing positions close to the theoretical optimum for face identification. We discuss future directions to investigate the functional basis of individual fixation behavior and face identity processing ability.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

