Bone marrow-confined IL-6 signaling mediates the progression of myelodysplastic syndromes to acute myeloid leukemia
- PMID: 35900794
- PMCID: PMC9435651
- DOI: 10.1172/JCI152673
Bone marrow-confined IL-6 signaling mediates the progression of myelodysplastic syndromes to acute myeloid leukemia
Abstract
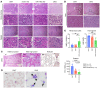
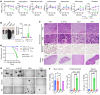
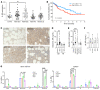
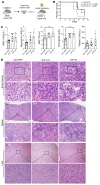
Myelodysplastic syndromes (MDS) are age-related myeloid neoplasms with increased risk of progression to acute myeloid leukemia (AML). The mechanisms of transformation of MDS to AML are poorly understood, especially in relation to the aging microenvironment. We previously established an mDia1/miR-146a double knockout (DKO) mouse model phenocopying MDS. These mice develop age-related pancytopenia with oversecretion of proinflammatory cytokines. Here, we found that most of the DKO mice underwent leukemic transformation at 12-14 months of age. These mice showed myeloblast replacement of fibrotic bone marrow and widespread leukemic infiltration. Strikingly, depletion of IL-6 in these mice largely rescued the leukemic transformation and markedly extended survival. Single-cell RNA sequencing analyses revealed that DKO leukemic mice had increased monocytic blasts that were reduced with IL-6 knockout. We further revealed that the levels of surface and soluble IL-6 receptor (IL-6R) in the bone marrow were significantly increased in high-risk MDS patients. Similarly, IL-6R was also highly expressed in older DKO mice. Blocking of IL-6 signaling significantly ameliorated AML progression in the DKO model and clonogenicity of CD34-positive cells from MDS patients. Our study establishes a mouse model of progression of age-related MDS to AML and indicates the clinical significance of targeting IL-6 signaling in treating high-risk MDS.
Keywords: Cancer; Hematology; Inflammation; Mouse models.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

