Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial
- PMID: 35900838
- PMCID: PMC9622299
- DOI: 10.1093/eurheartj/ehac401
Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial
Abstract
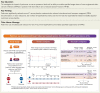
Aims: To investigate the impact of patiromer on the serum potassium level and its ability to enable specified target doses of renin-angiotensin-aldosterone system inhibitor (RAASi) use in patients with heart failure and reduced ejection fraction (HFrEF).
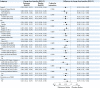
Methods and results: A total of 1642 patients with HFrEF and current or a history of RAASi-related hyperkalemia were screened and 1195 were enrolled in the run-in phase with patiromer and optimization of the RAASi therapy [≥50% recommended dose of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor, and 50 mg of mineralocorticoid receptor antagonist (MRA) spironolactone or eplerenone]. Specified target doses of the RAASi therapy were achieved in 878 (84.6%) patients; 439 were randomized to patiromer and 439 to placebo. All patients, physicians, and outcome assessors were blinded to treatment assignment. The primary endpoint was between-group difference in the adjusted mean change in serum potassium. Five hierarchical secondary endpoints were assessed. At the end of treatment, the median (interquartile range) duration of follow-up was 27 (13-43) weeks, the adjusted mean change in potassium was +0.03 mmol/l in the patiromer group and +0.13 mmol/l in the placebo group [difference in the adjusted mean change between patiromer and placebo: -0.10 mmol/l (95% confidence interval, CI -0.13, 0.07); P < 0.001]. Risk of hyperkalemia >5.5 mmol/l [hazard ratio (HR) 0.63; 95% CI 0.45, 0.87; P = 0.006), reduction of MRA dose (HR 0.62; 95% CI 0.45, 0.87; P = 0.006), and total adjusted hyperkalemia events/100 person-years (77.7 vs. 118.2; HR 0.66; 95% CI 0.53, 0.81; P < 0.001) were lower with patiromer. Hyperkalemia-related morbidity-adjusted events (win ratio 1.53, P < 0.001) and total RAASi use score (win ratio 1.25, P = 0.048) favored the patiromer arm. Adverse events were similar between groups.
Conclusion: Concurrent use of patiromer and high-dose MRAs reduces the risk of recurrent hyperkalemia (ClinicalTrials.gov: NCT03888066).
Keywords: Heart failure with reduced ejection fraction; Hyperkalemia; Patiromer; Potassium-binding polymer; Renin–angiotensin–aldosterone system inhibitor (RAASi).
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. T.F. reported consulting fees from Vifor Pharma, Bayer, Biosense Webster, CSL Behring, Galapagos, Minoryx, Novartis, LivaNova, Janssen, Roche, and honoraria from Fresenius Kabi. J.B. reported consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. G.F. reported honoraria from Bayer and Boehringer Ingelheim, committee membership for Medtronic, Vifor Pharma, Amgen, Servier, and Novartis, and grants from the European Commission. M.R.W. reported consulting fees from Vifor Pharma and AstraZeneca, and honoraria from Vifor Pharma. P.V.d.M. reported grants from Vifor Pharma, Pfizer, AstraZeneca, and Ionis, consulting fees from Vifor Pharma, Novartis and AstraZeneca, and honoraria from Vifor Pharma and Pharmacosmos. L.H.L. reported grants from AstraZeneca, Vifor, Boston Scientific, Boehringer Ingelheim, and Novartis, consulting fees from Merck, Vifor Pharma, AstraZeneca, Bayer, Pharmacosmos, MedScape, Sanofi, Lexicon, Myokardia, Boehringer Ingelheim, and Servier, honoraria from Abbott, MedScape, Radcliffe, AstraZeneca, and Novartis, and stock options and patents with AnaCardio. F.D. reported support and stock options from Vifor Pharma as a Vifor employee. F.P. reported consulting fees from Vifor Pharma and Novo Nordisk, honoraria from Servier, Pfizer, Novartis, and Boehringer Ingelheim, and presidency (unpaid) of the World Heart Federation. S.D.A. reports consulting fees from Abbott, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier, and Vifor Pharma, and grant support from Abbott and Vifor Pharma. M.M. reported consulting fees from Actelion, Amgen, AstraZeneca, Abbott Vascular, Bayer, Servier, Edwards Therapeutics, Livanova, Vifor Pharma, and WindTree Therapeutics. UMG reports grants, support and stock options from Vifor Pharma. M.K. reported consulting fees from Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Vifor Pharma, grants from AstraZeneca and Boehringer Ingelheim, and grant support from Abbott and Vifor Pharma, and honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. A.C. reported honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, Respicardia, and Viatris. P.R. reported grants from AstraZeneca, Bayer, Fresenius, Novartis, Vifor Fresenius Medical Care Renal Pharma, Relypsa, and Vifor, consulting fees from Bayer, Idorsia, G3P, KBP, and Sanofi, honoraria from Sequana medical, AstraZeneca, Bayer, Fresenius, Novartis, Grünenthal, Stealth Peptides, Vifor Fresenius Medical Care Renal, Novo Nordisk, Ablative Solutions, Corvidia, Relypsa, and Vifor CardioRenal, and non-financial support from Servier, Fresenius, and G3P. I.L.P. serves on an advisory board for Vifor Pharma Ltd and on the Steering Committee of the DIAMOND clinical trial. B.P. reported consulting fees from AstraZeneca, Boehringer Ingelheim/Lilly, Sanofi/Lexicon, SCPharmaceuticals, SQinnovations, G3 Pharmaceuticals, Sarfez, KBP biosciences, Cereno scientific, Phase bio, Proton Intel, and Vifor Pharma, stock options from SCPharmaceuticals, SQinnovations, G3 Pharmaceuticals, Sarfez, Cereno scientific, KBP biosciences, Proton Intel, and Vifor Pharma, US Patent 9931412- site specific delivery of eplerenone to the myocardium, and US Patent pending 63/045,783—Histone-modulating agents for the treatment and prevention of organ damage. No other authors reported disclosures.
Figures

Comment in
-
Potassium binders for patients with heart failure? The real enlightenment of the DIAMOND trial.Eur Heart J. 2022 Nov 1;43(41):4374-4377. doi: 10.1093/eurheartj/ehac399. Eur Heart J. 2022. PMID: 35900836 Free PMC article.
References
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. - PubMed
-
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. - PubMed
-
- Rossignol P, Duarte K, Girerd N, Karoui M, McMurray JJV, Swedberg K, et al. . Cardiovascular risk associated with serum potassium in the context of mineralocorticoid receptor antagonist use in patients with heart failure and left ventricular dysfunction. Eur J Heart Fail 2020;22:1402–1411. - PubMed
-
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin angiotensin-aldosterone system. N Engl J Med 2004;351:585–592. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

