miR-3156-5p is downregulated in serum of MEN1 patients and regulates expression of MORF4L2
- PMID: 35900839
- PMCID: PMC9422251
- DOI: 10.1530/ERC-22-0045
miR-3156-5p is downregulated in serum of MEN1 patients and regulates expression of MORF4L2
Abstract
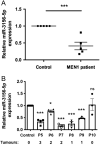
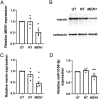
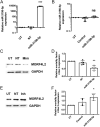
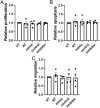
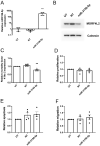
Multiple endocrine neoplasia type 1 (MEN1), caused by mutations in the MEN1 gene encoding menin, is an autosomal dominant disorder characterised by the combined occurrence of parathyroid, pituitary and pancreatic neuroendocrine tumours (NETs). Development of these tumours is associated with wide variations in their severity, order and ages (from <5 to >80 years), requiring life-long screening. To improve tumour surveillance and quality of life, better circulating biomarkers, particularly for pancreatic NETs that are associated with higher mortality, are required. We, therefore, examined the expression of circulating miRNA in the serum of MEN1 patients. Initial profiling analysis followed by qRT-PCR validation studies identified miR-3156-5p to be significantly downregulated (-1.3 to 5.8-fold, P < 0.05-0.0005) in nine MEN1 patients, compared to matched unaffected relatives. MEN1 knock-down experiments in BON-1 human pancreatic NET cells resulted in reduced MEN1 (49%, P < 0.05), menin (54%, P < 0.05) and miR-3156-5p expression (20%, P < 0.005), compared to control-treated cells, suggesting that miR-3156-5p downregulation is a consequence of loss of MEN1 expression. In silico analysis identified mortality factor 4-like 2 (MOR4FL2) as a potential target of miR-3156-5p, and in vitro functional studies in BON-1 cells transfected with either miR-3156-5p mimic or inhibitors showed that the miR-3156-5p mimic significantly reduced MORF4L2 protein expression (46%, P < 0.005), while miR-3156-5p inhibitor significantly increased MORF4L2 expression (1.5-fold, P < 0.05), compared to control-treated cells, thereby confirming that miR-3156-5p regulates MORF4L2 expression. Thus, the inverse relationship between miR-3156-5p and MORF4L2 expression represents a potential serum biomarker that could facilitate the detection of NET occurrence in MEN1 patients.
Keywords: menin; microRNA; mortality factor 4-like protein 2; multiple endocrine neoplasia type 1; neuroendocrine tumour.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

