Safety Monitoring of COVID-19 mRNA Vaccine Second Booster Doses Among Adults Aged ≥50 Years - United States, March 29, 2022-July 10, 2022
- PMID: 35900925
- PMCID: PMC9345177
- DOI: 10.15585/mmwr.mm7130a4
Safety Monitoring of COVID-19 mRNA Vaccine Second Booster Doses Among Adults Aged ≥50 Years - United States, March 29, 2022-July 10, 2022
Abstract
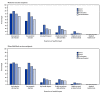
The Advisory Committee on Immunization Practices (ACIP) recommends that all persons aged ≥5 years receive 1 booster dose of a COVID-19 vaccine after completion of their primary series.* On March 29, 2022, the Food and Drug Administration (FDA) authorized a second mRNA booster dose ≥4 months after receipt of a first booster dose for adults aged ≥50 years and persons aged ≥12 years with moderate to severe immunocompromise (1,2). To characterize the safety of a second mRNA booster dose among persons aged ≥50 years, CDC reviewed adverse events and health impact assessments reported to v-safe and the Vaccine Adverse Event Reporting System (VAERS) after receipt of a second mRNA booster dose during March 29-July 10, 2022. V-safe is a voluntary smartphone-based U.S. active surveillance system that monitors adverse events occurring after COVID-19 vaccination. VAERS is a U.S. passive surveillance system for monitoring adverse events after vaccination, managed by CDC and FDA (3). During March 29-July 10, 2022, approximately 16.8 million persons in the United States aged ≥50 years received a fourth dose.† Among 286,380 v-safe registrants aged ≥50 years who reported receiving a second booster of an mRNA vaccine, 86.9% received vaccines from the same manufacturer for all 4 doses (i.e., homologous vaccination). Among registrants who reported homologous vaccination, injection site and systemic reactions were less frequent after the second booster dose than after the first booster dose. VAERS received 8,515 reports of adverse events after second mRNA booster doses among adults aged ≥50 years, including 8,073 (94.8%) nonserious and 442 (5.1%) serious events. CDC recommends that health care providers and patients be advised that local and systemic reactions are expected after a second booster dose, and that serious adverse events are uncommon.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Phillip G. Blanc reports previous membership on the alumni council (2017–2020) of the Harvard T.H. Chan School of Public Health and ownership of Community Health Systems, Inc. stock. No other potential conflicts of interest were disclosed.
Figures
References
-
- Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine letter of authorization (reissued). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/media/150386/download
-
- Food and Drug Administration. Moderna COVID-19 vaccine letter of authorization (reissued). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/144636/download
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

