USP22 suppresses the NLRP3 inflammasome by degrading NLRP3 via ATG5-dependent autophagy
- PMID: 35900990
- PMCID: PMC9980574
- DOI: 10.1080/15548627.2022.2107314
USP22 suppresses the NLRP3 inflammasome by degrading NLRP3 via ATG5-dependent autophagy
Abstract
The NLRP3 inflammasome is involved in a diverse range of inflammatory diseases. The activation of inflammasomes must be tightly regulated to prevent excessive inflammation, and the protein ubiquitination system is reported to be one of the ways in which inflammasome activation is regulated. However, the deubiquitination regulatory mechanisms of inflammasome activation remain elusive. Here, we demonstrated that USP22 (ubiquitin specific peptidase 22) promotes NLRP3 degradation and inhibits NLRP3 inflammasome activation. USP22 deficiency or in vivo silencing significantly increases alum-induced peritonitis and lipopolysaccharide-induced systemic inflammation. Mechanistically, USP22 inhibits NLRP3 inflammasome activation via the promotion of ATG5-mediated macroautophagy/autophagy. USP22 stabilizes ATG5 via decreasing K27- and K48-linked ubiquitination of ATG5 at the Lys118 site. Taken together, these findings reveal the role USP22 plays in the regulation of NLRP3 inflammasome activation and suggest a potential therapeutic target to treat NLRP3 inflammasome-related diseases.Abbreviations: ATG5: autophagy related 5; ATP: adenosine triphosphate; CASP1: caspase 1; IL18: interleukin 18; IL1B/IL-1β: interleukin 1 beta; LPS: lipopolysaccharide; NLRC4: NLR family, CARD domain containing 4; NLRP3: NLR family, pyrin domain containing 3; PYCARD/ASC: PYD and CARD domain containing; TNF/TNF-α: tumor necrosis factor; USP22: ubiquitin specific peptidase 22.
Keywords: Autophagy; NLRP3; autophagy related 5; inflammasome; ubiquitin specific peptidase 22.
Conflict of interest statement
All the authors declare that there are no conflicts of interest.
Figures


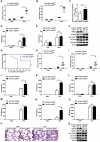
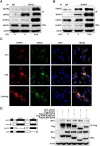
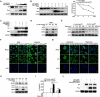

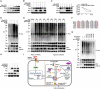
References
-
- Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
