Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: A multi-database, self-controlled case series study
- PMID: 35900992
- PMCID: PMC9333264
- DOI: 10.1371/journal.pmed.1004056
Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: A multi-database, self-controlled case series study
Abstract
Background: Myocarditis and pericarditis following the Coronavirus Disease 2019 (COVID-19) mRNA vaccines administration have been reported, but their frequency is still uncertain in the younger population. This study investigated the association between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) mRNA vaccines, BNT162b2, and mRNA-1273 and myocarditis/pericarditis in the population of vaccinated persons aged 12 to 39 years in Italy.
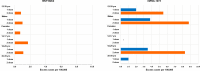
Methods and findings: We conducted a self-controlled case series study (SCCS) using national data on COVID-19 vaccination linked to emergency care/hospital discharge databases. The outcome was the first diagnosis of myocarditis/pericarditis between 27 December 2020 and 30 September 2021. Exposure risk period (0 to 21 days from the vaccination day, subdivided in 3 equal intervals) for first and second dose was compared with baseline period. The SCCS model, adapted to event-dependent exposures, was fitted using unbiased estimating equations to estimate relative incidences (RIs) and excess of cases (EC) per 100,000 vaccinated by dose, age, sex, and vaccine product. Calendar period was included as time-varying confounder in the model. During the study period 2,861,809 persons aged 12 to 39 years received mRNA vaccines (2,405,759 BNT162b2; 456,050 mRNA-1273); 441 participants developed myocarditis/pericarditis (346 BNT162b2; 95 mRNA-1273). Within the 21-day risk interval, 114 myocarditis/pericarditis events occurred, the RI was 1.99 (1.30 to 3.05) after second dose of BNT162b2 and 2.22 (1.00 to 4.91) and 2.63 (1.21 to 5.71) after first and second dose of mRNA-1273. During the [0 to 7) days risk period, an increased risk of myocarditis/pericarditis was observed after first dose of mRNA-1273, with RI of 6.55 (2.73 to 15.72), and after second dose of BNT162b2 and mRNA-1273, with RIs of 3.39 (2.02 to 5.68) and 7.59 (3.26 to 17.65). The number of EC for second dose of mRNA-1273 was 5.5 per 100,000 vaccinated (3.0 to 7.9). The highest risk was observed in males, at [0 to 7) days after first and second dose of mRNA-1273 with RI of 12.28 (4.09 to 36.83) and RI of 11.91 (3.88 to 36.53); the number of EC after the second dose of mRNA-1273 was 8.8 (4.9 to 12.9). Among those aged 12 to 17 years, the RI was of 5.74 (1.52 to 21.72) after second dose of BNT162b2; for this age group, the number of events was insufficient for estimating RIs after mRNA-1273. Among those aged 18 to 29 years, the RIs were 7.58 (2.62 to 21.94) after first dose of mRNA-1273 and 4.02 (1.81 to 8.91) and 9.58 (3.32 to 27.58) after second dose of BNT162b2 and mRNA-1273; the numbers of EC were 3.4 (1.1 to 6.0) and 8.6 (4.4 to 12.6) after first and second dose of mRNA-1273. The main study limitations were that the outcome was not validated through review of clinical records, and there was an absence of information on the length of hospitalization and, thus, the severity of the outcome.
Conclusions: This population-based study of about 3 millions of residents in Italy suggested that mRNA vaccines were associated with myocarditis/pericarditis in the population younger than 40 years. According to our results, increased risk of myocarditis/pericarditis was associated with the second dose of BNT162b2 and both doses of mRNA-1273. The highest risks were observed in males of 12 to 39 years and in males and females 18 to 29 years vaccinated with mRNA-1273. The public health implication of these findings should be considered in the light of the proven mRNA vaccine effectiveness in preventing serious COVID-19 disease and death.
Conflict of interest statement
I have read the journal’s policy and the author of this manuscript have the following competing interests: in the last 36 months, GT coordinated a pharmacoepi team at the University of Messina till Oct 2020 and currently at the academic spin-off INSPIRE that received research grants from PTC Therapeutics, Kiowa Kirin, Chiesi, Daiichi Sankyo for the conduct of observational studies on topics not related to the paper; GT participated to Advisory Board/interview sponsored by Eli Lilly, Amgen, Sanofi, SOBI, Gilead, ABBvie, Verpora and Daiichi Sankyo on topics not related to the paper.
Figures

References
-
- Centers for Disease Control and Prevention. COVID-19 VaST Technical Report—May 17, 2021. 2021. Available from: https://www.cdc.gov/vaccines/acip/work-groups-vast/technical-report-2021.... [cited 2022 Mar 1]
-
- European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 3–6 May 2021. 2021. Available from: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-r.... [cited 2022 Mar 1]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

