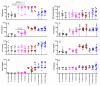
The percentage of CD39+ monocytes is higher in pregnant COVID-19+ patients than in nonpregnant COVID-19+ patients
- PMID: 35901034
- PMCID: PMC9333267
- DOI: 10.1371/journal.pone.0264566
The percentage of CD39+ monocytes is higher in pregnant COVID-19+ patients than in nonpregnant COVID-19+ patients
Abstract
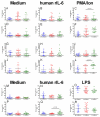
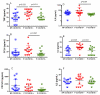
Current medical guidelines consider pregnant women with COVID-19 to be a high-risk group. Since physiological gestation downregulates the immunological response to maintain "maternal-fetal tolerance", SARS-CoV-2 infection may constitute a potentially threatening condition to both the mother and the fetus. To establish the immune profile in pregnant COVID-19+ patients, a cross-sectional study was conducted. Pregnant women with COVID-19 (P-COVID-19+; n = 15) were analyzed and compared with nonpregnant women with COVID-19 (NP-COVID-19+; n = 15) or those with physiological pregnancy (P-COVID-19-; n = 13). Serological cytokine and chemokine concentrations, leucocyte immunophenotypes, and mononuclear leucocyte responses to polyclonal stimuli were analyzed in all groups. Higher concentrations of serological TNF-α, IL-6, MIP1b and IL-4 were observed within the P-COVID-19+ group, while cytokines and chemokines secreted by peripheral leucocytes in response to LPS, IL-6 or PMA-ionomicin were similar among the groups. Immunophenotype analysis showed a lower percentage of HLA-DR+ monocytes in P-COVID-19+ than in P-COVID-19- and a higher percentage of CD39+ monocytes in P-COVID-19+ than in NP-COVID-19+. After whole blood polyclonal stimulation, similar percentages of T cells and TNF+ monocytes between groups were observed. Our results suggest that P-COVID-19+ elicits a strong inflammatory response similar to NP-COVID19+ but also displays an anti-inflammatory response that controls the ATP/adenosine balance and prevents hyperinflammatory damage in COVID-19.
Conflict of interest statement
All authors declare no competing interests.
Figures

References
-
- Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021. Epub 2021/04/23. doi: 10.1001/jamapediatrics.2021.1050 - DOI - PMC - PubMed
-
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–7. Epub 2020/11/06. doi: 10.15585/mmwr.mm6944e3 - DOI - PMC - PubMed
-
- Delahoy MJ, Whitaker M, O’Halloran A, Chai SJ, Kirley PD, Alden N, et al. Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19—COVID-NET, 13 States, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1347–54. Epub 2020/09/25. doi: 10.15585/mmwr.mm6938e1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

