N-acetyl cysteine prevents arecoline-inhibited C2C12 myoblast differentiation through ERK1/2 phosphorylation
- PMID: 35901044
- PMCID: PMC9333315
- DOI: 10.1371/journal.pone.0272231
N-acetyl cysteine prevents arecoline-inhibited C2C12 myoblast differentiation through ERK1/2 phosphorylation
Abstract
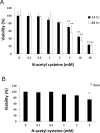
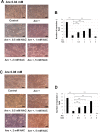
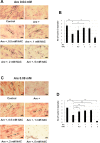
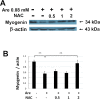
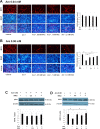
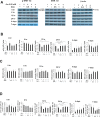
Arecoline is known to induce reactive oxygen species (ROS). Our previous studies showed that arecoline inhibited myogenic differentiation and acetylcholine receptor cluster formation of C2C12 myoblasts. N-acetyl-cysteine (NAC) is a known ROS scavenger. We hypothesize that NAC scavenges the excess ROS caused by arecoline. In this article we examined the effect of NAC on the inhibited myoblast differentiation by arecoline and related mechanisms. We found that NAC less than 2 mM is non-cytotoxic to C2C12 by viability analysis. We further demonstrated that NAC attenuated the decreased number of myotubes and nuclei in each myotube compared to arecoline treatment by H & E staining. We also showed that NAC prevented the decreased expression level of the myogenic markers, myogenin and MYH caused by arecoline, using immunocytochemistry and western blotting. Finally, we found that NAC restored the decreased expression level of p-ERK1/2 by arecoline. In conclusion, our results indicate that NAC attenuates the damage of the arecoline-inhibited C2C12 myoblast differentiation by the activation/phosphorylation of ERK. This is the first report to demonstrate that NAC has beneficial effects on skeletal muscle myogenesis through ERK1/2 upon arecoline treatment. Since defects of skeletal muscle associates with several diseases, NAC can be a potent drug candidate in diseases related to defects in skeletal muscle myogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Arecoline inhibits myogenic differentiation of C2C12 myoblasts by reducing STAT3 phosphorylation.Food Chem Toxicol. 2012 Oct;50(10):3433-9. doi: 10.1016/j.fct.2012.07.032. Epub 2012 Jul 28. Food Chem Toxicol. 2012. PMID: 22847137
-
Nilotinib impairs skeletal myogenesis by increasing myoblast proliferation.Skelet Muscle. 2018 Feb 20;8(1):5. doi: 10.1186/s13395-018-0150-5. Skelet Muscle. 2018. PMID: 29463296 Free PMC article.
-
Lactate induces C2C12 myoblasts differentiation by mediating ROS/p38 MAPK signalling pathway.Tissue Cell. 2024 Apr;87:102324. doi: 10.1016/j.tice.2024.102324. Epub 2024 Feb 12. Tissue Cell. 2024. PMID: 38354685
-
Arecoline inhibits and destabilizes agrin-induced acetylcholine receptor cluster formation in C2C12 myotubes.Food Chem Toxicol. 2013 Oct;60:391-6. doi: 10.1016/j.fct.2013.07.079. Epub 2013 Aug 8. Food Chem Toxicol. 2013. PMID: 23933062
-
Osteocalcin Induces Proliferation via Positive Activation of the PI3K/Akt, P38 MAPK Pathways and Promotes Differentiation Through Activation of the GPRC6A-ERK1/2 Pathway in C2C12 Myoblast Cells.Cell Physiol Biochem. 2017;43(3):1100-1112. doi: 10.1159/000481752. Epub 2017 Oct 5. Cell Physiol Biochem. 2017. PMID: 28977794
Cited by
-
The Controversial Roles of Areca Nut: Medicine or Toxin?Int J Mol Sci. 2023 May 19;24(10):8996. doi: 10.3390/ijms24108996. Int J Mol Sci. 2023. PMID: 37240342 Free PMC article. Review.
References
-
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, et al.. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem, 2004. 279(39): p. 41114–23. doi: 10.1074/jbc.M400674200 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

