Development, characterization and In-vitro evaluation of guar gum based new polymeric matrices for controlled delivery using metformin HCl as model drug
- PMID: 35901085
- PMCID: PMC9333214
- DOI: 10.1371/journal.pone.0271623
Development, characterization and In-vitro evaluation of guar gum based new polymeric matrices for controlled delivery using metformin HCl as model drug
Abstract
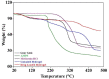
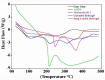
Currently, hydrogels are considered as ideal biomaterials due to their unique structure and characteristics that facilitates considerable hydrophilicity, swelling, drug loading and release. In this study, we report pH-responsive GG-MAA-AMPS hydrogel delivery system prepared via free radical polymerization technique. Hydrogels were loaded with Metformin HCl as a model drug. Hydrogels were characterized through Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and scanning electron microscopy (SEM). FTIR confirmed the successful crosslinking of reactants, hydrogel network formation and drug loading. TGA and DSC proved the higher thermal stability of reactants after crosslinking and drug loading. XRD analysis showed decrease in crystallinity of drug after loading into the hydrogels. SEM revealed smooth and glassy appearance of both loaded and unloaded hydrogels. Gel content was increased with increase in concentration of reactants. Drug entrapment was decreased by increasing concentration of GG and AMPS while MAA acted inversely. Hydrogels displayed pH-dependent swelling and drug release behavior being high at pH 6.8 and 7.4 while low at acidic pH (1.2). Oral tolerability in rabbits showed that hydrogels were safe without causing any hematological or histopathological changes in healthy rabbits. Based on the obtained results, GG-MAA-AMPS can be considered as potential carrier for metformin HCl as well as other hydrophilic drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Facile Synthesis of Chitosan Based-(AMPS-co-AA) Semi-IPNs as a Potential Drug Carrier: Enzymatic Degradation, Cytotoxicity, and Preliminary Safety Evaluation.Curr Drug Deliv. 2019;16(3):242-253. doi: 10.2174/1567201815666181024152101. Curr Drug Deliv. 2019. PMID: 30360742
-
Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin.Int J Pharm. 2019 Feb 10;556:236-245. doi: 10.1016/j.ijpharm.2018.12.020. Epub 2018 Dec 13. Int J Pharm. 2019. PMID: 30553956
-
Preparation of smart PVP/HPMC based IPN hydrogel, its characterization and toxicity evaluation.Pak J Pharm Sci. 2021 Sep;34(5(Supplementary)):1849-1859. Pak J Pharm Sci. 2021. PMID: 34836850
-
Controlled release of cephradine by biopolymers based target specific crosslinked hydrogels.Int J Biol Macromol. 2019 Jan;121:104-112. doi: 10.1016/j.ijbiomac.2018.10.018. Epub 2018 Oct 3. Int J Biol Macromol. 2019. PMID: 30291928
-
Hydrogels as promising carriers for the delivery of food bioactive ingredients.Front Nutr. 2022 Sep 27;9:1006520. doi: 10.3389/fnut.2022.1006520. eCollection 2022. Front Nutr. 2022. PMID: 36238460 Free PMC article. Review.
Cited by
-
Aluminum-Free Borosilicate Glass Functionalized Hydrogels for Enhanced Dental Tissue Regeneration.Materials (Basel). 2024 Nov 29;17(23):5862. doi: 10.3390/ma17235862. Materials (Basel). 2024. PMID: 39685297 Free PMC article.
-
Hydroxyethyl Cellulose-Based Hydrogels as Controlled Release Carriers for Amorphous Solid Dispersion of Bioactive Components of Radix Paeonia Alba.Molecules. 2023 Oct 28;28(21):7320. doi: 10.3390/molecules28217320. Molecules. 2023. PMID: 37959739 Free PMC article.
-
Synthesis and Evaluation of Rutin-Hydroxypropyl β-Cyclodextrin Inclusion Complexes Embedded in Xanthan Gum-Based (HPMC-g-AMPS) Hydrogels for Oral Controlled Drug Delivery.Antioxidants (Basel). 2023 Feb 22;12(3):552. doi: 10.3390/antiox12030552. Antioxidants (Basel). 2023. PMID: 36978800 Free PMC article.
-
Crosslinked PVA-g-poly(AMPS) Nanogels for Enhanced Solubility and Dissolution of Ticagrelor: Synthesis, Characterization, and Toxicity Evaluation.ACS Omega. 2024 May 2;9(19):21401-21415. doi: 10.1021/acsomega.4c01721. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764664 Free PMC article.
-
Preparation and Characterization of Theophylline Controlled Release Matrix System Incorporating Poloxamer 407, Stearyl Alcohol, and Hydroxypropyl Methylcellulose: A Novel Formulation and Development Study.Polymers (Basel). 2024 Feb 27;16(5):643. doi: 10.3390/polym16050643. Polymers (Basel). 2024. PMID: 38475326 Free PMC article.
References
-
- Kaith B, Jindal R, Kumar V, Bhatti MS. Optimal response surface design of Gum tragacanth-based poly [(acrylic acid)-co-acrylamide] IPN hydrogel for the controlled release of the antihypertensive drug losartan potassium. RSC advances. 2014;4(75):39822–9.
-
- Khan SA, Azam W, Ashames A, Fahelelbom KM, Ullah K, Mannan A, et al.. β-Cyclodextrin-based (IA-co-AMPS) Semi-IPNs as smart biomaterials for oral delivery of hydrophilic drugs: Synthesis, characterization, in-Vitro and in-Vivo evaluation. Journal of Drug Delivery Science and Technology. 2020;60:101970.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

