Effectiveness and safety of weekly paclitaxel and cetuximab as a salvage chemotherapy following immune checkpoint inhibitors for recurrent or metastatic head and neck squamous cell carcinoma: A multicenter clinical study
- PMID: 35901098
- PMCID: PMC9333293
- DOI: 10.1371/journal.pone.0271907
Effectiveness and safety of weekly paclitaxel and cetuximab as a salvage chemotherapy following immune checkpoint inhibitors for recurrent or metastatic head and neck squamous cell carcinoma: A multicenter clinical study
Erratum in
-
Correction: Effectiveness and safety of weekly paclitaxel and cetuximab as a salvage chemotherapy following immune checkpoint inhibitors for recurrent or metastatic head and neck squamous cell carcinoma: a multicenter clinical study.PLoS One. 2024 May 9;19(5):e0303720. doi: 10.1371/journal.pone.0303720. eCollection 2024. PLoS One. 2024. PMID: 38722895 Free PMC article.
Abstract
Objectives: The benefit of sequential therapy after immune checkpoint inhibitor (ICI) treatment for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) has been recently reported. Furthermore, there is a growing interest in the impact of cetuximab (Cmab)-containing salvage chemotherapy (SCT) and the therapeutic efficacy and adverse events (AEs) of Cmab administration prior to ICI administration.
Materials and methods: We retrospectively reviewed the medical records of 52 patients with R/M HNSCC treated with SCT (weekly paclitaxel [PTX], n = 7, or weekly PTX and Cmab [PC], n = 45).
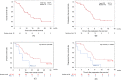
Results: The objective response rate (ORR) and a disease control rate (DCR) was 53.3% and 91.1% in the PC group and 42.9% and 57.1% in the PTX group, respectively. There was a significant difference in the DCR between the PC and PTX groups (p = 0.0143). The overall survival (OS) and progression-free survival were significantly better in the PC group than in the PTX group. On the other hand, the incidence of drug-induced interstitial pneumonia (DI-IP) in R/M HNSCC patients who received SCT was 21.2%. Patients in the PC group were divided according to whether they received Cmab (Group A) or did not receive Cmab (Group B) as palliative therapy prior to ICIs. Group B had a significantly better OS than Group A. Furthermore, our findings suggest that the incidence rate of DI-IP during SCT might be higher in Group B.
Conclusion: Although PC following ICIs shows dramatic efficacy, careful monitoring of AEs, including DI-IP, is recommended.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Clinical Outcomes of Cetuximab and Paclitaxel after Progression on Immune Checkpoint Inhibitors in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma.Medicina (Kaunas). 2021 Oct 23;57(11):1151. doi: 10.3390/medicina57111151. Medicina (Kaunas). 2021. PMID: 34833369 Free PMC article.
-
Clinical impact of weekly paclitaxel plus cetuximab is comparable to the EXTREME regimen for recurrent/metastatic head and neck squamous cell carcinoma.Int J Clin Oncol. 2021 Jul;26(7):1188-1195. doi: 10.1007/s10147-021-01907-x. Epub 2021 Apr 5. Int J Clin Oncol. 2021. PMID: 33821363
-
Cetuximab combined with paclitaxel or paclitaxel alone for patients with recurrent or metastatic head and neck squamous cell carcinoma progressing after EXTREME.Cancer Med. 2021 Jun;10(12):3952-3963. doi: 10.1002/cam4.3953. Epub 2021 May 25. Cancer Med. 2021. PMID: 34080776 Free PMC article.
-
Combination therapy with immune checkpoint inhibitors in recurrent or metastatic squamous cell carcinoma of the head and neck: A meta-analysis.Int Immunopharmacol. 2023 Jun;119:110270. doi: 10.1016/j.intimp.2023.110270. Epub 2023 May 5. Int Immunopharmacol. 2023. PMID: 37150013 Review.
-
Efficacy and safety of immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: A systematic review and meta-analysis of randomized clinical trials.Cancer Med. 2023 Oct;12(20):20277-20286. doi: 10.1002/cam4.6564. Epub 2023 Oct 10. Cancer Med. 2023. PMID: 37814950 Free PMC article.
Cited by
-
Survival impact of sequential chemotherapy following pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma.Int J Clin Oncol. 2024 Jun;29(6):764-770. doi: 10.1007/s10147-024-02508-0. Epub 2024 Mar 30. Int J Clin Oncol. 2024. PMID: 38555323
-
Correction: Effectiveness and safety of weekly paclitaxel and cetuximab as a salvage chemotherapy following immune checkpoint inhibitors for recurrent or metastatic head and neck squamous cell carcinoma: a multicenter clinical study.PLoS One. 2024 May 9;19(5):e0303720. doi: 10.1371/journal.pone.0303720. eCollection 2024. PLoS One. 2024. PMID: 38722895 Free PMC article.
-
Combined Positive Score and Cisplatin Sensitivity Are Prognostic Factors for Response to Nivolumab Therapy for Recurrent Metastatic Squamous Cell Carcinoma of the Head and Neck.Clin Med Insights Oncol. 2024 Oct 16;18:11795549241290030. doi: 10.1177/11795549241290030. eCollection 2024. Clin Med Insights Oncol. 2024. PMID: 39429682 Free PMC article.
-
Chemotherapy following immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: clinical effectiveness and influence of inflammatory and nutritional factors.Discov Oncol. 2023 Aug 29;14(1):158. doi: 10.1007/s12672-023-00774-4. Discov Oncol. 2023. PMID: 37642856 Free PMC article.
References
-
- Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al.. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine. 2016;375(19):1856–67. Epub 2016/10/11. doi: 10.1056/NEJMoa1602252 ; PubMed Central PMCID: PMC5564292. - DOI - PMC - PubMed
-
- Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al.. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;393(10167):156–67. Epub 2018/12/05. doi: 10.1016/S0140-6736(18)31999-8 . - DOI - PubMed
-
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al.. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;394(10212):1915–28. Epub 2019/11/05. doi: 10.1016/S0140-6736(19)32591-7 . - DOI - PubMed
-
- Matsuki T, Okamoto I, Fushimi C, Takahashi H, Okada T, Kondo T, et al.. Real-World, Long-Term Outcomes of Nivolumab Therapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck and Impact of the Magnitude of Best Overall Response: A Retrospective Multicenter Study of 88 Patients. Cancers. 2020;12(11). Epub 2020/11/22. doi: 10.3390/cancers12113427 ; PubMed Central PMCID: PMC7699139. - DOI - PMC - PubMed
-
- Yen CJ, Kiyota N, Hanai N, Takahashi S, Yokota T, Iwae S, et al.. Two-year follow-up of a randomized phase III clinical trial of nivolumab vs. the investigator’s choice of therapy in the Asian population for recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141). Head & neck. 2020;42(10):2852–62. Epub 2020/06/26. doi: 10.1002/hed.26331 ; PubMed Central PMCID: PMC7540331. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

