Furanoditerpenes from Spongia (Spongia) tubulifera Display Mitochondrial-Mediated Neuroprotective Effects by Targeting Cyclophilin D
- PMID: 35901231
- PMCID: PMC9686139
- DOI: 10.1021/acschemneuro.2c00208
Furanoditerpenes from Spongia (Spongia) tubulifera Display Mitochondrial-Mediated Neuroprotective Effects by Targeting Cyclophilin D
Abstract
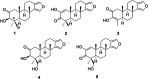
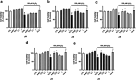
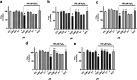
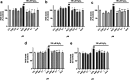
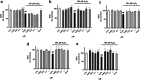
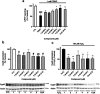
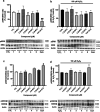
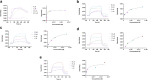
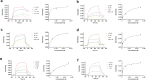
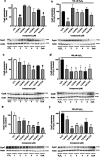
Neuroprotective properties of five previously described furanoditerpenes 1-5, isolated from Spongia (Spongia) tubulifera, were evaluated in an in vitro oxidative stress model in SH-SY5Y cells. Dose-response treatments revealed that 1-5 improved cell survival at nanomolar concentrations through the restoration of mitochondrial membrane potential and the reduction of reactive oxygen species. Their ability to prevent the mitochondrial permeability transition pore opening was also assessed, finding that 4 and 5 inhibited the channel at 0.001 μM. This inhibition was accompanied by a decrease in the expression of cyclophilin D, the main regulator of the pore, which was also reduced by 1 and 2. However, the activation of ERK and GSK3β, upstream modulators of the channel, was not affected by compounds. Therefore, their ability to bind cyclophilin D was evaluated by surface plasmon resonance, observing that 2-5 presented equilibrium dissociation constants in the micromolar range. All compounds also showed affinity for cyclophilin A, being 1 selective toward this isoform, while 2 and 5 exhibited selectivity for cyclophilin D. When the effects on the intracellular expression of cyclophilins A-C were determined, it was found that only 1 decreased cyclophilin A, while cyclophilins B and C were diminished by most compounds, displaying enhanced effects under oxidative stress conditions. Results indicate that furanoditerpenes 1-5 have mitochondrial-mediated neuroprotective properties through direct interaction with cyclophilin D. Due to the important role of this protein in oxidative stress and inflammation, compounds are promising drugs for new therapeutic strategies against neurodegeneration.
Keywords: Spongia (Spongia) tubulifera; cyclophilin; furanoditerpenes; mitochondria; neuroprotection; oxidative stress.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Pagano G.; Pallardó F. V.; Lyakhovich A.; Tiano L.; Fittipaldi M. R.; Toscanesi M.; Trifuoggi M. Aging-Related Disorders and Mitochondrial Dysfunction: A Critical Review for Prospect Mitoprotective Strategies Based on Mitochondrial Nutrient Mixtures. Int. J. Mol. Sci. 2020, 21, 706010.3390/ijms21197060. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

