Deconstructive Synthesis of Bridged and Fused Rings via Transition-Metal-Catalyzed "Cut-and-Sew" Reactions of Benzocyclobutenones and Cyclobutanones
- PMID: 35901263
- PMCID: PMC9386905
- DOI: 10.1021/acs.accounts.2c00400
Deconstructive Synthesis of Bridged and Fused Rings via Transition-Metal-Catalyzed "Cut-and-Sew" Reactions of Benzocyclobutenones and Cyclobutanones
Abstract
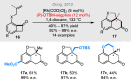
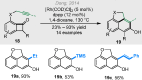
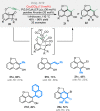
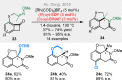
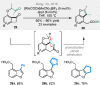
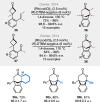
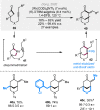
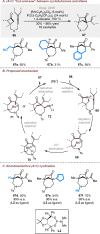
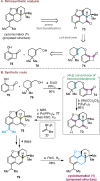
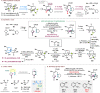
Bridged and fused rings are commonly found in biologically important molecules. Current tactics to construct these ring systems are primarily based on stepwise ring formation (i.e., making one ring first followed by making another) and cycloaddition reactions (e.g., Diels-Alder reaction). To seek a complementary and perhaps more unified ring-forming approach, a deconstructive strategy based on C-C bond activation of cyclic ketones has been conceived. The named "cut-and-sew" reaction uses cyclic ketones with a tethered unsaturated moiety as substrates, which involves oxidative addition of a transition metal into the ketone C-C bond followed by intramolecular insertion of the unsaturated unit. This strategy has proved successful to access diverse ring scaffolds that are nontrivial to construct otherwise.This Account offers a concise summary of our laboratory's systematic efforts in developing transition metal-catalyzed cut-and-sew reactions for the synthesis of bridged and fused rings over the past 10 years. In particular, we will focus on the reactions using readily available benzocyclobutenones and cyclobutanones. To date, the scope of the cut-and-sew reactions has been greatly expanded. First, diverse unsaturated moieties can serve as suitable coupling partners, such as alkenyl, alkynyl, allenyl, carbonyl, and iminyl groups. Second, a variety of reaction modes have been uncovered. In this account, (4 + 2), (4 + 2 - 1), and (4 + 1) cycloadditions that lead to a range of bridged or fused scaffolds will be summarized. Third, enantioselective transformations have been realized to efficiently construct chiral scaffolds, which are enabled by two strategies: enantio-determining migratory insertion and desymmetrization of cyclobutanones. Fourth, the synthetic applications have been demonstrated in streamlined total syntheses of a number of complex natural products. Compared to conventional synthetic logics, the cut-and-sew reaction allows the development of new bond-disconnecting strategies. Thus, the syntheses of (-)-cycloclavine, (-)-thebainone A, penicibilaenes, and the proposed cycloinumakiol are discussed in more detail.In addition to the narrative of the development of the cut-and-sew chemistry, this Account also aims to provide core guiding foundations and inspirations toward broader deconstructive synthetic applications through C-C bond cleavage. It is anticipated that more classes of cyclic compounds could serve as the substrates beyond benzocyclobutenones and cyclobutanones, and more diverse unsaturated moieties could be coupled. It can also be envisaged that more innovative utilization of this cut-and-sew strategy in complex organic syntheses will be revealed in the near future.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. - DOI - PubMed
- Lovering F. Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Comm 2013, 4, 515–519. 10.1039/c2md20347b. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

