The innovation of the symbiosome has enhanced the evolutionary stability of nitrogen fixation in legumes
- PMID: 35901264
- PMCID: PMC9541511
- DOI: 10.1111/nph.18321
The innovation of the symbiosome has enhanced the evolutionary stability of nitrogen fixation in legumes
Abstract
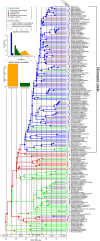
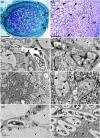
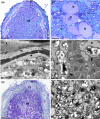
Nitrogen-fixing symbiosis is globally important in ecosystem functioning and agriculture, yet the evolutionary history of nodulation remains the focus of considerable debate. Recent evidence suggesting a single origin of nodulation followed by massive parallel evolutionary losses raises questions about why a few lineages in the N2 -fixing clade retained nodulation and diversified as stable nodulators, while most did not. Within legumes, nodulation is restricted to the two most diverse subfamilies, Papilionoideae and Caesalpinioideae, which show stable retention of nodulation across their core clades. We characterize two nodule anatomy types across 128 species in 56 of the 152 genera of the legume subfamily Caesalpinioideae: fixation thread nodules (FTs), where nitrogen-fixing bacteroids are retained within the apoplast in modified infection threads, and symbiosomes, where rhizobia are symplastically internalized in the host cell cytoplasm within membrane-bound symbiosomes (SYMs). Using a robust phylogenomic tree based on 997 genes from 147 Caesalpinioideae genera, we show that losses of nodulation are more prevalent in lineages with FTs than those with SYMs. We propose that evolution of the symbiosome allows for a more intimate and enduring symbiosis through tighter compartmentalization of their rhizobial microsymbionts, resulting in greater evolutionary stability of nodulation across this species-rich pantropical legume clade.
Keywords: Leguminosae; evolution; fixation threads; nitrogen fixation; nodulation; phylogenomics; symbiosis; symbiosomes.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Figures

References
-
- Ardley J, Sprent J. 2021. Evolution and biogeography of actinorhizal plants and legumes: a comparison. Journal of Ecology 109: 1098–1121.
-
- Batterman SA, Hedin LO, Van Breugel M, Ransijn J, Craven DJ, Hall JS. 2013. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502: 224–227. - PubMed
-
- Brewin NJ. 2004. Plant cell wall remodelling in the rhizobium–legume symbiosis. Critical Reviews in Plant Sciences 23: 293–316.
-
- Bruneau A, Mercure M, Lewis GP, Herendeen PS. 2008. Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 86: 697–718.
-
- Cardoso D, De Queiroz LP, Toby Pennington R, De Lima HC, Fonty É, Wojciechowski MF, Lavin M. 2012. Revisiting the phylogeny of papilionoid legumes: new insights from comprehensively sampled early‐branching lineages. American Journal of Botany 99: 1991–2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

