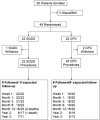
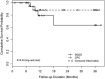
Outcomes of the Second Aqueous Shunt Implant Versus Transscleral Cyclophotocoagulation Treatment Study: A Randomized Comparative Trial
- PMID: 35901309
- PMCID: PMC9415215
- DOI: 10.1097/IJG.0000000000002079
Outcomes of the Second Aqueous Shunt Implant Versus Transscleral Cyclophotocoagulation Treatment Study: A Randomized Comparative Trial
Abstract
Prcis: Short-term overall success rates were high with either SGDD or CPC. However, SGDD was associated with more clinic visits and an increased risk of additional glaucoma surgery. Both treatments were reasonable options for eyes with inadequately controlled IOP after a single GDD.
Purpose: The purpose of this study is to compare the implantation of a second glaucoma drainage device (SGDD) and transscleral cyclophotocoagulation (CPC) in eyes with inadequately controlled intraocular pressure (IOP), despite the presence of a preexisting glaucoma drainage device.
Methods: Patients with inadequately controlled IOP, despite the medical therapy and a preexisting glaucoma drainage device, were enrolled at 14 clinical centers and randomly assigned to treatment with a SGDD or CPC.
Main outcome measures: Surgical failure was defined as: (1) IOP ≤5 mm Hg or >18 mm Hg or <20% reduction below baseline on maximum tolerated topical ocular hypotensive therapy, (2) reoperation for glaucoma, or (3) loss of light perception. The primary outcome measure was overall success with or without adjunctive medical therapy.
Results: Forty-two eyes of 42 participants were randomized to SGDD (n=22) or CPC (n=20). Mean duration of follow-up was 18.6 (±12.1; range: 1.1-38.6) months. The cumulative success rate was 79% for SGDD and 88% for CPC at 1 year ( P =0.63). Although the study was underpowered, no significant differences in IOP, postoperative number of IOP-lowering medications, or adverse events were observed. The number of additional glaucoma surgeries ( P =0.003), office visits during the first 3 months ( P <0.001), and office visits per month after month 3 ( P <0.001) were greater in the SGDD group.
Conclusions: Short-term overall success rates were high with either SGDD or CPC. However, SGDD was associated with more clinic visits and an increased risk of additional glaucoma surgery.
Trial registration: ClinicalTrials.gov NCT02691455.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Disclosure: Dr R.L.G. reports personal fees from Aerie during the conduct of the study; membership of Data and Safety Monitoring Board for Glauckos and consultant and stockholder for Intelligent Retinal Imaging Systems, outside the submitted work. Dr D.S.G. reports personal fees (consultant) from Allergan, Alcon, Aerie, and Eyenovia outside the submitted work. Dr L.R.P. reports grants from NIH, The Glaucoma Foundation, and Research to Prevent Blindness, as well as personal fees from Eyenovia, Twenty-Twenty, and Skye Biosciences outside the submitted work. Dr S.L.M. reports grants from AbbVie outside the submitted work. Dr A.P.T. reports grants or contracts from Google and Research to Prevent Blindness (to Northwestern University); consulting fees from Ivantis, Sandoz, and Zeiss; and payment for expert testimony from Ivantis. Dr R.M.F. reports personal fees from Bausch and Lomb and Catawba; stock in 4DMD; and grants from Santen and Ivantis outside the submitted work. The remaining authors declare no conflict of interest.
Figures
References
-
- Arora KS, Robin AL, Corcoran KJ, et al. . Use of various glaucoma surgeries and procedures in Medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122:1615–1624. - PubMed
-
- Christakis PG, Kalenak JW, Tsai JC, et al. . The Ahmed Versus Baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123:2093–2102. - PubMed
-
- Anand A, Tello C, Sidoti PA, et al. . Sequential glaucoma implants in refractory glaucoma. Am J Ophthalmol. 2010;149:95–101. - PubMed