Newly Substituted Anilino-Fluoroquinolones with Proliferation Inhibition Potential against a Panel of Cancer Cell Lines
- PMID: 35901360
- PMCID: PMC9727339
- DOI: 10.31557/APJCP.2022.23.7.2507
Newly Substituted Anilino-Fluoroquinolones with Proliferation Inhibition Potential against a Panel of Cancer Cell Lines
Abstract
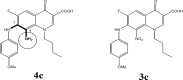
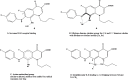
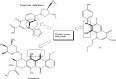
Background: From a chemistry point of view, we hypothesized that superlative dual cytotoxicity-radical scavenging bioefficacies of series 4 FQs correlate to their acidic groups and C8-C7 ethylene diamine Chelation Bridge.
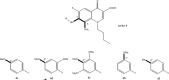
Methodology: Newly synthesized 16 lipophilic-acid chelating FQs have been screened for in vitro duality of proliferation inhibition and radical scavenging capacities.
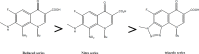
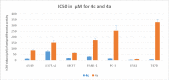
Results: Substantially in LPS prompted RAW264.7 macrophages inflammation, IC50 values (µM) in the ascending order of new FQs' NO scavenging/antiinflammation capacity were 4e<4b<3d<4f<5c0.05). In comparison to classical and robust antineoplastic agent cisplatin and unlike triazoloFQs; nitroFQs (3a, 3b and 3f) and the reduced FQs (4a, 4c, 4d and 4e) exerted antiproliferation IC50 values <50 µM in leukaemia K562. Besides nitroFQ 3, the reduced FQs (4c and 4f) exhibited antineoplastic IC50 values <50 µM in lung A549 carcinoma. NitroFQ 3c and reduced FQs 4b, 4c, and 4f in breast MCF7 and reduced 4c in pancreatic PANC1 had reduction of viability IC50 values <50 µM. NitroFQ 3e, reduced FQs 4b and, 4c and triazoloFQ 5a exerted antiproliferation IC50 values <50 µM in breast T47D cells. Also nitroFQ 3e, reduced FQ 4c and triazoloFQ 5f exhibited antineoplastic IC50 values <50 µM in PC3 prostate cancer cells. Exceptionally triazoloFQ 5a, but neither nitro- nor reduced FQs, had cytotoxicity IC50 value <50 µM in resistant melanoma A375 cells. Unequivocally 4b antineoplastic effectiveness linked with its radical scavenging and antiinflammation effects while 3d and 5c lacked matching antiproliferation potentialities to their exquisite antiinflammation capacities. Explicitly reduced 4e and 4f exerted antiinflammation-selective cytotoxicity duality in vitro.
Conclusion: Collectively, this work reveals lipophilic-acidic chelator FQs as authentic agents for the repurposing approach in anticancer chemotherapy/prevention.
Keywords: Antiinflammation and antiCancer; Cisplatin; Fluoroquinolones and Triazoloquinolones; NO-radical scavenging; Sulphorodhamine B and Greiss assay.
Conflict of interest statement
Authors declare no conflict of interest
Figures
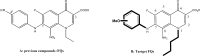
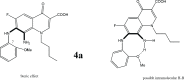
References
-
- AbdulFattah T, Saeed A, Al-Hiari YM, et al. Functionalized Furo[3,2-c] coumarins as Anti-proliferative, Anti-lipolytic, and Antiinflammatory Compounds: Synthesis and Molecular Docking Studies. J Mol Struc. 2019;1179:390–400.
-
- Alabsi Y, Al-Hiari Y, Kasabri V, et al. In vitro modulation of pancreatic lipase and proliferation of obesity related-colorectal cancer cell line panel by novel synthetic FQs. Rev Roum Chim. 2018;63:1123–34.
-
- AlHallaq T, Al-Hiari Y, Kasabri V, et al. In vitro Antiproliferative Properties of Lipophililic -Acid Chelating Fluoroquinolones and triazoloFluoroquinolones with 7-dihaloanilinosubstitution. AntiCancer Agents Med Chem. 2022 Accepted. - PubMed
-
- AlKhalil M, Al-Hiari Y, Kasabri V, et al. Selected pharmacotherapy agents as antiproliferative and antiinflammatory compounds. Drug Dev Res. 2020;81:470–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

