Highly Stereospecific Cyclizations of Homoallylic Silanols
- PMID: 35901375
- PMCID: PMC10019461
- DOI: 10.1021/acs.joc.2c01170
Highly Stereospecific Cyclizations of Homoallylic Silanols
Abstract
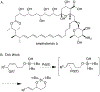
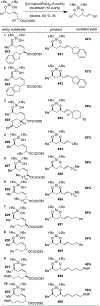
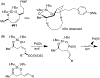
We demonstrate that di-tert-butylsilanols are competent nucleophiles for the intramolecular interception of palladium π-allyl species. In these reactions, allyl ethyl carbonates are the best precursors for the formation of palladium π-allyl intermediates, and [(Cinnamyl)PdCl]2/BINAP is superior to other Pd salt/ligand framework combinations. Our optimized protocol is compatible with a variety of silanol substrates. Importantly, the cyclization is perfectly stereospecific, proceeding via an anti-syn mechanism, which stands in contrast to reported analogous reactions of alcohols and phenols, known to proceed via an anti-anti mechanism. The alkenes in the product dioxasilinanes serve as blank slates for further functionalization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Correa A; García Mancheño O; Bolm C Iron-catalysed carbon–heteroatom and heteroatom–heteroatom bond forming processes. Chem. Soc. Rev 2008, 37, 1108–1117. - PubMed
-
- Hosoya K; Odagi M; Nagasawa K Guanidine organocatalysis for enantioselective carbon-heteroatom bond-forming reactions. Tetrahedron Lett. 2018, 59, 687–696.
-
- Puleo TR; Sujansky SJ; Wright SE; Bandar JS Organic Superbases in Recent Synthetic Methodology Research. Chem. - Eur. J 2021, 27, 4216–4229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

