Arousal and salience network connectivity alterations in surgical temporal lobe epilepsy
- PMID: 35901709
- PMCID: PMC10127440
- DOI: 10.3171/2022.5.JNS22837
Arousal and salience network connectivity alterations in surgical temporal lobe epilepsy
Abstract
Objective: It is poorly understood why patients with mesial temporal lobe epilepsy (TLE) have cognitive deficits and brain network changes that extend beyond the temporal lobe, including altered extratemporal intrinsic connectivity networks (ICNs). However, subcortical arousal structures project broadly to the neocortex, are affected by TLE, and thus may contribute to these widespread network effects. The authors' objective was to examine functional connectivity (FC) patterns between subcortical arousal structures and neocortical ICNs, possible neurocognitive relationships, and FC changes after epilepsy surgery.
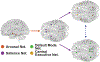
Methods: The authors obtained resting-state functional magnetic resonance imaging (fMRI) in 50 adults with TLE and 50 controls. They compared nondirected FC (correlation) and directed FC (Granger causality laterality index) within the salience network, default mode network, and central executive network, as well as between subcortical arousal structures; these 3 ICNs were also compared between patients and controls. They also used an fMRI-based vigilance index to relate alertness to arousal center FC. Finally, fMRI was repeated in 29 patients > 12 months after temporal lobe resection.
Results: Nondirected FC within the salience (p = 0.042) and default mode (p = 0.0008) networks, but not the central executive network (p = 0.79), was decreased in patients in comparison with controls (t-tests, corrected). Nondirected FC between the salience network and subcortical arousal structures (nucleus basalis of Meynert, thalamic centromedian nucleus, and brainstem pedunculopontine nucleus) was reduced in patients in comparison with controls (p = 0.0028-0.015, t-tests, corrected), and some of these connectivity abnormalities were associated with lower processing speed index, verbal comprehension, and full-scale IQ. Interestingly, directed connectivity measures suggested a loss of top-down influence from the salience network to the arousal nuclei in patients. After resection, certain FC patterns between the arousal nuclei and salience network moved toward control values in the patients, suggesting that some postoperative recovery may be possible. Although an fMRI-based vigilance measure suggested that patients exhibited reduced alertness over time, FC abnormalities between the salience network and arousal structures were not influenced by the alertness levels during the scans.
Conclusions: FC abnormalities between subcortical arousal structures and ICNs, such as the salience network, may be related to certain neurocognitive deficits in TLE patients. Although TLE patients demonstrated vigilance abnormalities, baseline FC perturbations between the arousal and salience networks are unlikely to be driven solely by alertness level, and some may improve after surgery. Examination of the arousal network and ICN disturbances may improve our understanding of the downstream clinical effects of TLE.
Keywords: arousal structures; epilepsy surgery; fMRI; focal epilepsy; functional connectivity; networks; neuroimaging; temporal lobe epilepsy.
Conflict of interest statement
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures


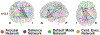

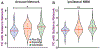
References
-
- Serafini A, Kuate C, Gelisse P, et al. Sleep before and after temporal lobe epilepsy surgery. Seizure. 2012; 21(4): 260–265. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

