Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases
- PMID: 35901858
- PMCID: PMC10550198
- DOI: 10.1016/j.jconrel.2022.07.022
Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases
Abstract
Treatment of neurological lysosomal storage disorders (LSDs) are limited because of impermeability of the blood-brain barrier (BBB) to macromolecules. Nanoformulations targeting BBB transcytosis are being explored, but the status of these routes in LSDs is unknown. We studied nanocarriers (NCs) targeted to the transferrin receptor (TfR), ganglioside GM1 or ICAM1, associated to the clathrin, caveolar or cell adhesion molecule (CAM) routes, respectively. We used brain endothelial cells and mouse models of acid sphingomyelinase-deficient Niemann Pick disease (NPD), and postmortem LSD patients' brains, all compared to respective controls. NC transcytosis across brain endothelial cells and brain distribution in mice were affected, yet through different mechanisms. Reduced TfR and clathrin expression were found, along with decreased transcytosis in cells and mouse brain distribution. Caveolin-1 expression and GM1 transcytosis were also reduced, yet increased GM1 levels seemed to compensate, providing similar NC brain distribution in NPD vs. control mice. A tendency to lower NHE-1 levels was seen, but highly increased ICAM1 expression in cells and human brains correlated with increased transcytosis and brain distribution in mice. Thus, transcytosis-related alterations in NPD and likely other LSDs may impact therapeutic access to the brain, illustrating the need for these mechanistic studies.
Keywords: Blood-brain barrier; Lysosomal storage disorders; Neurological diseases; Targeted nanocarriers; Transcytosis pathways.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
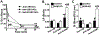



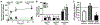
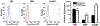
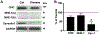

References
-
- Saraiva C; Praça C; Ferreira R; Santos T; Ferreira L; Bernardino L, Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. Journal of Controlled Release 2016, 235, 34–47. - PubMed
-
- Johnsen KB; Burkhart A; Thomsen LB; Andresen TL; Moos T, Targeting the transferrin receptor for brain drug delivery. Progress in Neurobiology 2019, 181, 101665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

