Age-related changes in the organization of spontaneously occurring behaviors
- PMID: 35901935
- PMCID: PMC10436331
- DOI: 10.1016/j.beproc.2022.104713
Age-related changes in the organization of spontaneously occurring behaviors
Abstract
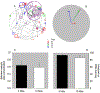
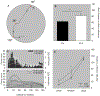
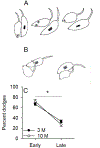
Age-related changes in spatial and temporal processing have been documented across a range of species. Rodent studies typically investigate differences in performance between adult and senescent animals; however, progressive loss of neurons in the hippocampus and cortex has been observed to occur as early as after adolescence. Therefore, the current study evaluated the effects of age in three- and ten-month-old female rats on the organization of movement in open field and food protection behaviors, two tasks that have previously dissociated hippocampal and cortical pathology. Age-related differences were observed in general measures of locomotion, spatial orientation, and attentional processing. The results of the current study are consistent with age-related changes in the processing of spatial information and motivation that occur earlier in life than previously anticipated. These observations establish a foundation for future studies evaluating interventions that influence these age-related differences in performance.
Keywords: Exploration; Food protection; Movement kinematics; Open field; Rats; Senescence.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures




References
-
- Ayyildiz M, Kozan R, Agar E, & Kaplan S (2008). Sexual dimorphism in the medial vestibular nucleus of adult rats: stereological study. Anatomical science international, 83(3), 131–139. - PubMed
-
- Banovetz MT, Lake RI, Blackwell AA, Oltmanns JRO, Schaeffer EA, Yoder RM, & Wallace DG (2021). Effects of acquired vestibular pathology on the organization of mouse exploratory behavior. Experimental Brain Research, 1–15. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

