Acute Kidney Injury in Patients with Liver Disease
- PMID: 35902128
- PMCID: PMC9718035
- DOI: 10.2215/CJN.03040322
Acute Kidney Injury in Patients with Liver Disease
Abstract
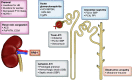
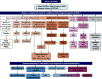
AKI is commonly encountered in patients with decompensated cirrhosis, and it is associated with unfavorable outcomes. Among factors specific to cirrhosis, hepatorenal syndrome type 1, also referred to as hepatorenal syndrome-AKI, is the most salient and unique etiology. Patients with cirrhosis are vulnerable to traditional causes of AKI, such as prerenal azotemia, acute tubular injury, and acute interstitial nephritis. In addition, other less common etiologies of AKI specifically related to chronic liver disease should be considered, including abdominal compartment syndrome, cardiorenal processes linked to cirrhotic cardiomyopathy and portopulmonary hypertension, and cholemic nephropathy. Furthermore, certain types of GN can cause AKI in cirrhosis, such as IgA nephropathy or viral hepatitis related. Therefore, a comprehensive diagnostic approach is needed to evaluate patients with cirrhosis presenting with AKI. Management should be tailored to the specific underlying etiology. Albumin-based volume resuscitation is recommended in prerenal AKI. Acute tubular injury and acute interstitial nephritis are managed with supportive care, withdrawal of the offending agent, and, potentially, corticosteroids in acute interstitial nephritis. Short of liver transplantation, vasoconstrictor therapy is the primary treatment for hepatorenal syndrome type 1. Timing of initiation of vasoconstrictors, the rise in mean arterial pressure, and the degree of cholestasis are among the factors that determine vasoconstrictor responsiveness. Large-volume paracentesis and diuretics are indicated to relieve intra-abdominal hypertension and renal vein congestion. Direct-acting antivirals with or without immunosuppression are used to treat hepatitis B/C-associated GN. In summary, AKI in cirrhosis requires careful consideration of multiple potentially pathogenic factors and the implementation of targeted therapeutic interventions.
Keywords: Critical Care Nephrology and Acute Kidney Injury Series; acute kidney injury; cirrhosis; hepatorenal syndrome; liver failure.
Copyright © 2022 by the American Society of Nephrology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

