Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of nonmelanoma skin cancer
- PMID: 35902131
- PMCID: PMC9341183
- DOI: 10.1136/jitc-2021-004434
Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of nonmelanoma skin cancer
Abstract
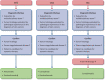
Nonmelanoma skin cancers (NMSCs) are some of the most commonly diagnosed malignancies. In general, early-stage NMSCs have favorable outcomes; however, a small subset of patients develop resistant, advanced, or metastatic disease, or aggressive subtypes that are more challenging to treat successfully. Recently, immune checkpoint inhibitors (ICIs) have been approved by the US Food and Drug Administration (FDA) for the treatment of Merkel cell carcinoma (MCC), cutaneous squamous cell carcinoma (CSCC), and basal cell carcinoma (BCC). Although ICIs have demonstrated activity against NMSCs, the routine clinical use of these agents may be more challenging due to a number of factors including the lack of predictive biomarkers, the need to consider special patient populations, the management of toxicity, and the assessment of atypical responses. With the goal of improving patient care by providing expert guidance to the oncology community, the Society for Immunotherapy of Cancer (SITC) convened a multidisciplinary panel of experts to develop a clinical practice guideline (CPG). The expert panel drew on the published literature as well as their own clinical experience to develop recommendations for healthcare professionals on important aspects of immunotherapeutic treatment for NMSCs, including staging, biomarker testing, patient selection, therapy selection, post-treatment response evaluation and surveillance, and patient quality of life (QOL) considerations, among others. The evidence- and consensus-based recommendations in this CPG are intended to provide guidance to cancer care professionals treating patients with NMSCs.
Keywords: clinical trials as topic; guidelines as topic; immunotherapy; skin neoplasms.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CAB: consulting fees: Regeneron; contracted research: Alpha Tau Medical, Merck, Amgen, Elekta, and EMD Serono. SB: consulting fees: Genentech, Bristol Myers Squibb, EMD Serono, Sanofi Genzyme, and Exicure; contracted research: Bristol Myers Squibb, EMD Serono, Immune Design, Merck, Novartis, Oncosec, Nantkwest, Exicure, and Nektar. KBB: consulting fees: Iovance Biotherapeutics and Nektar Therapeutics. SC: consulting fees: Bristol Myers Squibb, Regeneron, Sanofi Genzyme, Novartis, EMD Serono, Exicure, and Pfizer; contracted research: Bristol Myers Squibb, Merck, Regeneron, Sanofi Genzyme, Novartis, EMD Serono, Exicure, and Pfizer. ZE: consulting fees: Regeneron, Genentech, Novartis, Natera, Pfizer, and OncoSec (advisory boards); research funding (to institution): Pfizer and Novartis. BRG: consulting fees: Quest Imaging (consultant/advisor) and Castle Biosciences (speaker); contracted research: NIT and Alkermes (research grant). KLK: contracted research: Merck, Bristol Myers Squibb, Karyopharm, Novartis, GlaxoSmithKline, Immunocore, and Iovance. HK: consulting fees: Nektar, Roche-Genentech, Pfizer, Iovance, Immunocore, Celldex, Array Biopharma, Merck, Elevate Bio, Instil Bio, and Bristol Myers Squibb; contracted research: Merck, Bristol Myers Squibb, and Apexigen (institutional research grants (to institution)). EJL: consulting fees: Array BioPharma, Bristol Myers Squibb, Eisai, EMD Serono, Genentech, MacroGenics, Novartis, Merck, Odonate Therapeutics, OncoSec, Regeneron, and Sanofi Genzyme; contracted research: Bristol Myers Squibb, Merck, and Regeneron. KM: consulting fees: Pfizer (advisory board). DMM: consulting fees: Sanofi, Regeneron, Intellisphere LLC, OncLive, and Checkpoint Therapeutics; ownership interest: Checkpoint Therapeutics. PN: consulting fees: EMD Serono, Pfizer, 4SC, Wolters Kluwer Health, Merck, Sanofi Genzyme, and Regeneron; contracted research: EMD Serono and Bristol Myers Squibb; other: secretary–treasurer for Society of Investigative Dermatology; partner consulting fees: Pfizer and Kadmon; partner contracted research: Millenium Pharmaceuticals, Amgen, Novartis, Kadmon, Pfizer, Syndax Pharmaceuticals, and Incyte; partner other: President of American Society of Hematology. ACP: consulting fees: Regeneron, Bristol Myers Squibb, and Merck; fees for non-CE services: Bristol Myers Squibb; contracted research: Merck, Bristol Myers Squibb, Iovance, Regeneron, Takeda, Ideaya, and Replimune (all payments to institution). IP: consulting fees: Merck, Amgen, Nouscom, Nektar, and Iovance; other: Bristol Myers Squibb (medical writing/article processing charges); ownership interest: Celldex. GR: consulting fees: Sanofi Genzyme, Regeneron, EMD Serono, Pfizer, Castle, and Merck; ownership interest: Regeneron and Syros Pharmaceuticals. ESR: consulting fees: Sanofi, Leo Pharma, Checkpoint Therapeutics, Pellepharm, and Jounce. AWS: consulting fees: Bristol Myers Squibb, Merck, and EMD Serono. VKS: consulting fees: Merck, Regeneron, and Eisai; other: Bristol Myers Squibb and Polynoma (data safety monitoring boards). MTT: consulting fees: Nanostring LLC, Bristol Myers Squibb, Merck, Myriad Genetics, and Seattle Genetics. EAT: nothing to disclose. IB: Nothing to disclose. Society for Immunotherapy of Cancer (SITC) staff: SMW - shares owned: Pacific Biosciences of California Inc and Editas Medicine Inc; ME, CG, EG, and AK - nothing to disclose.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
