Reshaping the tumor microenvironment with oncolytic viruses, positive regulation of the immune synapse, and blockade of the immunosuppressive oncometabolic circuitry
- PMID: 35902132
- PMCID: PMC9341188
- DOI: 10.1136/jitc-2022-004935
Reshaping the tumor microenvironment with oncolytic viruses, positive regulation of the immune synapse, and blockade of the immunosuppressive oncometabolic circuitry
Abstract
Background: Oncolytic viruses are considered part of immunotherapy and have shown promise in preclinical experiments and clinical trials. Results from these studies have suggested that tumor microenvironment remodeling is required to achieve an effective response in solid tumors. Here, we assess the extent to which targeting specific mechanisms underlying the immunosuppressive tumor microenvironment optimizes viroimmunotherapy.
Methods: We used RNA-seq analyses to analyze the transcriptome, and validated the results using Q-PCR, flow cytometry, and immunofluorescence. Viral activity was analyzed by replication assays and viral titration. Kyn and Trp metabolite levels were quantified using liquid chromatography-mass spectrometry. Aryl hydrocarbon receptor (AhR) activation was analyzed by examination of promoter activity. Therapeutic efficacy was assessed by tumor histopathology and survival in syngeneic murine models of gliomas, including Indoleamine 2,3-dioxygenase (IDO)-/- mice. Flow cytometry was used for immunophenotyping and quantification of cell populations. Immune activation was examined in co-cultures of immune and cancer cells. T-cell depletion was used to identify the role played by specific cell populations. Rechallenge experiments were performed to identify the development of anti-tumor memory.
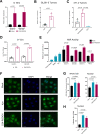
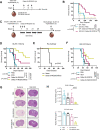
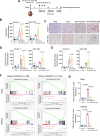
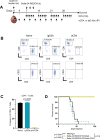
Results: Bulk RNA-seq analyses showed the activation of the immunosuppressive IDO-kynurenine-AhR circuitry in response to Delta-24-RGDOX infection of tumors. To overcome the effect of this pivotal pathway, we combined Delta-24-RGDOX with clinically relevant IDO inhibitors. The combination therapy increased the frequency of CD8+ T cells and decreased the rate of myeloid-derived suppressor cell and immunosupressive Treg tumor populations in animal models of solid tumors. Functional studies demonstrated that IDO-blockade-dependent activation of immune cells against tumor antigens could be reversed by the oncometabolite kynurenine. The concurrent targeting of the effectors and suppressors of the tumor immune landscape significantly prolonged the survival in animal models of orthotopic gliomas.
Conclusions: Our data identified for the first time the in vivo role of IDO-dependent immunosuppressive pathways in the resistance of solid tumors to oncolytic adenoviruses. Specifically, the IDO-Kyn-AhR activity was responsible for the resurface of local immunosuppression and resistance to therapy, which was ablated through IDO inhibition. Our data indicate that combined molecular and immune therapy may improve outcomes in human gliomas and other cancers treated with virotherapy.
Keywords: Brain Neoplasms; Immunomodulation; Oncolytic Virotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MA, HJ, FL, CG-M, and JF report license agreements with DNAtrix. CG-M and JF are shareholders of DNAtrix. MA reports DNAtrix-sponsored research not related to this work.
Figures


Similar articles
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Oncolytic HSV-IL27 expression improves CD8 T cell function and therapeutic activity in syngeneic glioma models.J Immunother Cancer. 2025 Jul 7;13(7):e012227. doi: 10.1136/jitc-2025-012227. J Immunother Cancer. 2025. PMID: 40623717 Free PMC article.
-
IDO1-AhR axis increases T regulatory cells in Plasmodium vivax malaria infection.Front Immunol. 2025 Jul 14;16:1474447. doi: 10.3389/fimmu.2025.1474447. eCollection 2025. Front Immunol. 2025. PMID: 40726977 Free PMC article.
-
Oncolytic immunovirotherapy: finding the tumor antigen needle in the antiviral haystack.Immunotherapy. 2025 Jun;17(8):585-594. doi: 10.1080/1750743X.2025.2513853. Epub 2025 Jun 6. Immunotherapy. 2025. PMID: 40474818 Review.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
Cited by
-
Novel immunogenic cell death-related risk signature to predict prognosis and immune microenvironment in lung adenocarcinoma.J Cancer Res Clin Oncol. 2023 Jan;149(1):307-323. doi: 10.1007/s00432-022-04555-4. Epub 2022 Dec 28. J Cancer Res Clin Oncol. 2023. PMID: 36575346 Free PMC article.
-
Lymphopenia associated with whole-brain radiotherapy and its effects on clinical outcomes of patients with brain metastases.Sci Rep. 2024 Sep 12;14(1):21315. doi: 10.1038/s41598-024-71943-w. Sci Rep. 2024. PMID: 39266704 Free PMC article.
-
Targeting the Kynurenine Pathway: A Novel Approach in Tumor Therapy.Expert Rev Mol Med. 2025 Mar 5;27:1-33. doi: 10.1017/erm.2025.5. Online ahead of print. Expert Rev Mol Med. 2025. PMID: 40040508 Free PMC article. Review.
-
Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response.Front Cell Infect Microbiol. 2023 Mar 15;13:1142172. doi: 10.3389/fcimb.2023.1142172. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37009515 Free PMC article. Review.
-
Unveiling the Immune effects of AHR in tumors: a decade of insights from bibliometric analysis (2010-2023).Discov Oncol. 2024 Nov 4;15(1):616. doi: 10.1007/s12672-024-01480-5. Discov Oncol. 2024. PMID: 39495340 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
