Induction of severe hypoxemia and low lung recruitability for the evaluation of therapeutic ventilation strategies: a translational model of combined surfactant-depletion and ventilator-induced lung injury
- PMID: 35902450
- PMCID: PMC9334469
- DOI: 10.1186/s40635-022-00456-5
Induction of severe hypoxemia and low lung recruitability for the evaluation of therapeutic ventilation strategies: a translational model of combined surfactant-depletion and ventilator-induced lung injury
Abstract
Background: Models of hypoxemic lung injury caused by lavage-induced pulmonary surfactant depletion are prone to prompt recovery of blood oxygenation following recruitment maneuvers and have limited translational validity. We hypothesized that addition of injurious ventilation following surfactant-depletion creates a model of the acute respiratory distress syndrome (ARDS) with persistently low recruitability and higher levels of titrated "best" positive end-expiratory pressure (PEEP) during protective ventilation.
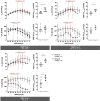
Methods: Two types of porcine lung injury were induced by lung lavage and 3 h of either protective or injurious ventilation, followed by 3 h of protective ventilation (N = 6 per group). Recruitment maneuvers (RM) and decremental PEEP trials comparing oxygenation versus dynamic compliance were performed after lavage and at 3 h intervals of ventilation. Pulmonary gas exchange function, respiratory mechanics, and ventilator-derived parameters were assessed after each RM to map the course of injury severity and recruitability.
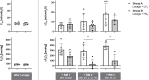
Results: Lung lavage impaired respiratory system compliance (Crs) and produced arterial oxygen tensions (PaO2) of 84±13 and 80±15 (FIO2 = 1.0) with prompt increase after RM to 270-395 mmHg in both groups. After subsequent 3 h of either protective or injurious ventilation, PaO2/FIO2 was 104±26 vs. 154±123 and increased to 369±132 vs. 167±87 mmHg in response to RM, respectively. After additional 3 h of protective ventilation, PaO2/FIO2 was 120±15 vs. 128±37 and increased to 470±68 vs. 185±129 mmHg in response to RM, respectively. Subsequently, decremental PEEP titration revealed that Crs peaked at 36 ± 10 vs. 25 ± 5 ml/cm H2O with PEEP of 12 vs. 16 cmH2O, and PaO2/FIO2 peaked at 563 ± 83 vs. 334 ± 148 mm Hg with PEEP of 16 vs. 22 cmH2O in the protective vs. injurious ventilation groups, respectively. The large disparity of recruitability between groups was not reflected in the Crs nor the magnitude of mechanical power present after injurious ventilation, once protective ventilation was resumed.
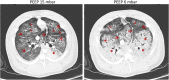
Conclusion: Addition of transitory injurious ventilation after lung lavage causes prolonged acute lung injury with diffuse alveolar damage and low recruitability yielding high titrated PEEP levels. Mimicking lung mechanical and functional characteristics of ARDS, this porcine model rectifies the constraints of single-hit lavage models and may enhance the translation of experimental research on mechanical ventilation strategies.
Keywords: Acute lung injury; Acute respiratory distress syndrome; Closed-loop ventilation; Mechanical power; Recruitment maneuver; Surfactant depletion; Ventilator-induced lung injury.
© 2022. The Author(s).
Conflict of interest statement
RK is an employee of EKU Elektronik GmbH, Germany. LH and WB are employees of Fritz Stephan GmbH, Germany. Otherwise, the authors have no competing interests.
Figures




References
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
-
- Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM, Acute Lung Injury in Animals Study G An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

