Therapeutic Effect of IL-21 Blockage by Gene Therapy in Experimental Autoimmune Encephalomyelitis
- PMID: 35902536
- PMCID: PMC9606180
- DOI: 10.1007/s13311-022-01279-8
Therapeutic Effect of IL-21 Blockage by Gene Therapy in Experimental Autoimmune Encephalomyelitis
Abstract
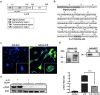
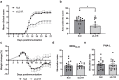
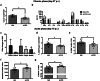
The pathogenic role of the interleukin 21 (IL-21) in different autoimmune diseases, such as multiple sclerosis (MS), has been extensively studied. However, its pleiotropic nature makes it a cytokine that may exhibit different activity depending on the immunological stage of the disease. In this study, we developed a gene therapy strategy to block the interaction between IL-21 and its receptor (IL-21R) by using adeno-associated vectors (AAV) encoding a new soluble cytokine receptor (sIL21R) protein. We tested this strategy in a murine model of experimental autoimmune encephalomyelitis (EAE), obtaining different clinical effects depending on the time at which the treatment was applied. Although the administration of the treatment during the development of the immune response was counterproductive, the preventive administration of the therapeutic vectors showed a protective effect by reducing the number of animals that developed the disease, as well as an improvement at the histopathological level and a modification of the immunological profile of the animals treated with the AAV8.sIL21R. The beneficial effect of the treatment was also observed when inducing the expression of the therapeutic molecule once the first neurological signs were established in a therapeutic approach with a doxycyline (Dox)-inducible expression system. All these clinical results highlight the pleiotropicity of this cytokine in the different clinical stages and its key role in the EAE immunopathogenesis.
Keywords: AAV; EAE; IL-21; Multiple sclerosis; Soluble receptor.
© 2022. The Author(s).
Figures




References
-
- Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:1–27. - PubMed
-
- Traugott U, Reinherz EL, Raine CS. Multiple sclerosis. Distribution of T cells, T cell subsets and Ia-positive macrophages in lesions of different ages. J Neuroimmunol. 1983;4:201–221. - PubMed
-
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

