PAK6 promotes homologous-recombination to enhance chemoresistance to oxaliplatin through ATR/CHK1 signaling in gastric cancer
- PMID: 35902562
- PMCID: PMC9334622
- DOI: 10.1038/s41419-022-05118-8
PAK6 promotes homologous-recombination to enhance chemoresistance to oxaliplatin through ATR/CHK1 signaling in gastric cancer
Abstract
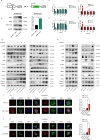
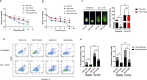
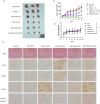
Chemoresistance remains the primary challenge of clinical treatment of gastric cancer (GC), making the biomarkers of chemoresistance crucial for treatment decision. Our previous study has reported that p21-actived kinase 6 (PAK6) is a prognostic factor for selecting which patients with GC are resistant to 5-fluorouracil/oxaliplatin chemotherapy. However, the mechanistic role of PAK6 in chemosensitivity remains unknown. The present study identified PAK6 as an important modulator of the DNA damage response (DDR) and chemosensitivity in GC. Analysis of specimens from patients revealed significant associations between the expression of PAK6 and poorer stages, deeper invasion, more lymph node metastases, higher recurrence rates, and resistance to oxaliplatin. Cells exhibited chemosensitivity to oxaliplatin after knockdown of PAK6, but showed more resistant to oxaliplatin when overexpressing PAK6. Functionally, PAK6 mediates cancer chemoresistance by enhancing homologous recombination (HR) to facilitate the DNA double-strand break repair. Mechanistically, PAK6 moves into nucleus to promote the activation of ATR, thereby further activating downstream repair protein CHK1 and recruiting RAD51 from cytoplasm to the DNA damaged site to repair the broken DNA in GC. Activation of ATR is the necessary step for PAK6 mediated HR repair to protect GC cells from oxaliplatin-induced apoptosis, and ATR inhibitor (AZD6738) could block the PAK6-mediated HR repair, thereby reversing the resistance to oxaliplatin and even promoting the sensitivity to oxaliplatin regardless of high expression of PAK6. In conclusion, these findings indicate a novel regulatory mechanism of PAK6 in modulating the DDR and chemoresistance in GC and provide a reversal suggestion in clinical decision.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Prognostic and Predictive Value of p21-activated Kinase 6 Associated Support Vector Machine Classifier in Gastric Cancer Treated by 5-fluorouracil/Oxaliplatin Chemotherapy.EBioMedicine. 2017 Aug;22:78-88. doi: 10.1016/j.ebiom.2017.06.028. Epub 2017 Jul 1. EBioMedicine. 2017. PMID: 28687498 Free PMC article.
-
Prevention of DNA Replication Stress by CHK1 Leads to Chemoresistance Despite a DNA Repair Defect in Homologous Recombination in Breast Cancer.Cells. 2020 Jan 17;9(1):238. doi: 10.3390/cells9010238. Cells. 2020. PMID: 31963582 Free PMC article.
-
Upregulation of CRABP2 by TET1-mediated DNA hydroxymethylation attenuates mitochondrial apoptosis and promotes oxaliplatin resistance in gastric cancer.Cell Death Dis. 2022 Oct 4;13(10):848. doi: 10.1038/s41419-022-05299-2. Cell Death Dis. 2022. PMID: 36195596 Free PMC article.
-
An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update.Cell Cycle. 2021 Sep;20(18):1760-1784. doi: 10.1080/15384101.2021.1966584. Epub 2021 Aug 26. Cell Cycle. 2021. PMID: 34437813 Free PMC article. Review.
-
ATR signaling in mammalian meiosis: From upstream scaffolds to downstream signaling.Environ Mol Mutagen. 2020 Aug;61(7):752-766. doi: 10.1002/em.22401. Epub 2020 Aug 13. Environ Mol Mutagen. 2020. PMID: 32725817 Free PMC article. Review.
Cited by
-
Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity.Biomedicines. 2023 Feb 5;11(2):462. doi: 10.3390/biomedicines11020462. Biomedicines. 2023. PMID: 36830998 Free PMC article. Review.
-
Transcriptomic response of overexpression ZNF32 in breast cancer cells.Sci Rep. 2024 Nov 18;14(1):28407. doi: 10.1038/s41598-024-80125-7. Sci Rep. 2024. PMID: 39557972 Free PMC article.
-
Multi-Pathway Study for Oxaliplatin Resistance Reduction.Curr Issues Mol Biol. 2025 Mar 4;47(3):172. doi: 10.3390/cimb47030172. Curr Issues Mol Biol. 2025. PMID: 40136426 Free PMC article. Review.
-
Urinary exosome proteins PAK6 and EGFR as noninvasive diagnostic biomarkers of diabetic nephropathy.BMC Nephrol. 2023 Oct 3;24(1):291. doi: 10.1186/s12882-023-03343-7. BMC Nephrol. 2023. PMID: 37789280 Free PMC article.
-
EZH1/2 plays critical roles in oocyte meiosis prophase I in mice.Biol Res. 2024 Nov 8;57(1):83. doi: 10.1186/s40659-024-00564-4. Biol Res. 2024. PMID: 39511641 Free PMC article.
References
-
- Al-Batran S-E, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–42. doi: 10.1200/JCO.2007.13.9378. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

