PACAP-PAC1R modulates fear extinction via the ventromedial hypothalamus
- PMID: 35902577
- PMCID: PMC9334354
- DOI: 10.1038/s41467-022-31442-w
PACAP-PAC1R modulates fear extinction via the ventromedial hypothalamus
Abstract
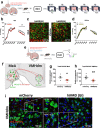
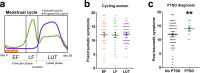
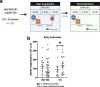
Exposure to traumatic stress can lead to fear dysregulation, which has been associated with posttraumatic stress disorder (PTSD). Previous work showed that a polymorphism in the PACAP-PAC1R (pituitary adenylate cyclase-activating polypeptide) system is associated with PTSD risk in women, and PACAP (ADCYAP1)-PAC1R (ADCYAP1R1) are highly expressed in the hypothalamus. Here, we show that female mice subjected to acute stress immobilization (IMO) have fear extinction impairments related to Adcyap1 and Adcyap1r1 mRNA upregulation in the hypothalamus, PACAP-c-Fos downregulation in the Medial Amygdala (MeA), and PACAP-FosB/ΔFosB upregulation in the Ventromedial Hypothalamus dorsomedial part (VMHdm). DREADD-mediated inhibition of MeA neurons projecting to the VMHdm during IMO rescues both PACAP upregulation in VMHdm and the fear extinction impairment. We also found that women with the risk genotype of ADCYAP1R1 rs2267735 polymorphism have impaired fear extinction.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013).
-
- Bryant, R. A. Posttraumatic stress disorder. in The Science of Cognitive Behavioral Therapy (eds Hofmann, S. & Asmundson, G.) 319–336 (Elsevier, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

