Staphylococcus aureus increases platelet reactivity in patients with infective endocarditis
- PMID: 35902612
- PMCID: PMC9334290
- DOI: 10.1038/s41598-022-16681-7
Staphylococcus aureus increases platelet reactivity in patients with infective endocarditis
Abstract
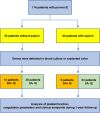
Thromboembolism is frequent in infective endocarditis (IE). However, the optimal antithrombotic regimen in IE is unknown. Staphylococcus aureus (SA) is the leading cause of IE. First studies emphasize increased platelet reactivity by SA. In this pilot study, we hypothesized that platelet reactivity is increased in patients with SA- IE, which could be abrogated by antiplatelet medication. We conducted a prospective, observatory, single-center cohort study in 114 patients with IE, with four cohorts: (1) SA coagulase positive IE without aspirin (ASA) medication, (2) coagulase negative IE without ASA, (3) SA coagulase positive IE with ASA, (4) coagulase negative IE with ASA. Platelet function was measured by Multiplate electrode aggregometry, blood clotting by ROTEM thromboelastometry. Bleeding events were assessed according to TIMI classification. In ASA-naïve patients, aggregation with ADP was increased with coag. pos. IE (coagulase negative: 39.47 ± 4.13 AUC vs. coagulase positive: 59.46 ± 8.19 AUC, p = 0.0219). This was abrogated with ASA medication (coagulase negative: 42.4 ± 4.67 AUC vs. coagulase positive: 45.11 ± 6.063 AUC p = 0.7824). Aspirin did not increase bleeding in SA positive patients. However, in SA negative patients with aspirin, red blood cell transfusions were enhanced. SA coagulase positive IE is associated with increased platelet reactivity. This could be abrogated by aspirin without increased bleeding risk. The results of this pilot study suggest that ASA might be beneficial in SA coagulase positive IE. This needs to be confirmed in clinical trials.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG, Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009;169(5):463–473. doi: 10.1001/archinternmed.2008.603. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

