Developing and testing a Corona VaccinE tRiAL pLatform (COVERALL) to study Covid-19 vaccine response in immunocompromised patients
- PMID: 35902817
- PMCID: PMC9330973
- DOI: 10.1186/s12879-022-07621-x
Developing and testing a Corona VaccinE tRiAL pLatform (COVERALL) to study Covid-19 vaccine response in immunocompromised patients
Abstract
Background: The rapid course of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic calls for fast implementation of clinical trials to assess the effects of new treatment and prophylactic interventions. Building trial platforms embedded in existing data infrastructures is an ideal way to address such questions within well-defined subpopulations.
Methods: We developed a trial platform building on the infrastructure of two established national cohort studies: the Swiss human immunodeficiency virus (HIV) Cohort Study (SHCS) and Swiss Transplant Cohort Study (STCS). In a pilot trial, termed Corona VaccinE tRiAL pLatform (COVERALL), we assessed the vaccine efficacy of the first two licensed SARS-CoV-2 vaccines in Switzerland and the functionality of the trial platform.
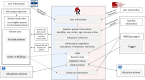
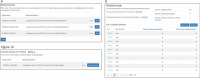
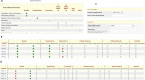
Results: Using Research Electronic Data Capture (REDCap), we developed a trial platform integrating the infrastructure of the SHCS and STCS. An algorithm identifying eligible patients, as well as baseline data transfer ensured a fast inclusion procedure for eligible patients. We implemented convenient re-directions between the different data entry systems to ensure intuitive data entry for the participating study personnel. The trial platform, including a randomization algorithm ensuring balance among different subgroups, was continuously adapted to changing guidelines concerning vaccination policies. We were able to randomize and vaccinate the first trial participant the same day we received ethics approval. Time to enroll and randomize our target sample size of 380 patients was 22 days.
Conclusion: Taking the best of each system, we were able to flag eligible patients, transfer patient information automatically, randomize and enroll the patients in an easy workflow, decreasing the administrative burden usually associated with a trial of this size.
Keywords: HIV; Immunocompromised; REDCap; SARS-CoV-2; Transplant patients; Trial platform.
© 2022. The Author(s).
Conflict of interest statement
KK, DS, JS, MO, MK, MB, BS and FC have no conflicts of interest. HCB has received in the 36 months prior to the submission of this manuscript grants, support for travelling, consultancy fees and honorarium from Gilead, BMS, Viiv Healthcare, Roche and Pfizer that were not related to this project. He serves as the president of the association contre le HIV et autres infections transmissibles. In this function, he has received support for the Swiss HIV Cohort Study from ViiV Healthcare, Gilead, BMS, and MSD. HFG, outside of this study, reports grants from the Swiss National Science Foundation, National Institutes of Health (NIH), and the Swiss HIV Cohort Study, unrestricted research grants from Gilead Sciences, Roche, and Yvonne Jacob Foundation, personal fees from consulting or advisory boards or data safety monitoring boards for Merck, Gilead Sciences, ViiV Healthcare, Mepha, and Sandoz. HFG’s institution received money for participation in the following clinical Covid-19 studies: 540-7773/5774 (Gilead), TICO (ACTIV-3, INSIGHT/NIH), and the Morningsky 435 study (Roche).
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

