RUNX1T1 function in cell fate
- PMID: 35902872
- PMCID: PMC9330642
- DOI: 10.1186/s13287-022-03074-w
RUNX1T1 function in cell fate
Abstract
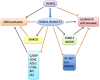
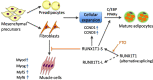
RUNX1T1 (Runt-related transcription factor 1, translocated to 1), a myeloid translocation gene (MTG) family member, is usually investigated as part of the fusion protein RUNX1-RUNX1T1 for its role in acute myeloid leukemia. In the main, by recruiting histone deacetylases, RUNX1T1 negatively influences transcription, enabling it to regulate the proliferation and differentiation of hematopoietic progenitors. Moreover, the formation of blood vessels, neuronal differentiation, microglial activation following injury, and intestinal development all relate closely to the expression of RUNX1T1. Furthermore, through alternative splicing of RUNX1T1, short and long isoforms have been noted to mediate adipogenesis by balancing the differentiation and proliferation of adipocytes. In addition, RUNX1T1 plays wide-ranging and diverse roles in carcinoma as a biomarker, suppressor, or positive regulator of carcinogenesis, closely correlated to specific organs and dominant signaling pathways. The aim of this work was to investigate the structure of RUNX1T1, which contains four conserved nervy homolog domains, and to demonstrate crosstalk with the Notch signaling pathway. Moreover, we endeavored to illustrate the effects of RUNX1T1 on cell fate from multiple aspects, including its influence on hematopoiesis, neuronal differentiation, microglial activation, intestinal development, adipogenesis, angiogenesis, and carcinogenesis.
Keywords: Cell fate; Development; Differentiation; Progenitor cells; RUNX1T1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Erickson PF, Robinson M, Owens G, Drabkin HA. The ETO portion of acute myeloid leukemia t(8;21) fusion transcript encodes a highly evolutionarily conserved, putative transcription factor. Cancer Res. 1994;54(7):1782–1786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

