Olfactory swab sampling optimization for α-synuclein aggregate detection in patients with Parkinson's disease
- PMID: 35902902
- PMCID: PMC9330656
- DOI: 10.1186/s40035-022-00311-3
Olfactory swab sampling optimization for α-synuclein aggregate detection in patients with Parkinson's disease
Erratum in
-
Correction: Olfactory swab sampling optimization for α-synuclein aggregate detection in patients with Parkinson's disease.Transl Neurodegener. 2022 Aug 12;11(1):38. doi: 10.1186/s40035-022-00312-2. Transl Neurodegener. 2022. PMID: 35962443 Free PMC article. No abstract available.
Abstract
Background: In patients with Parkinson's disease (PD), real-time quaking-induced conversion (RT-QuIC) detection of pathological α-synuclein (α-syn) in olfactory mucosa (OM) is not as accurate as in other α-synucleinopathies. It is unknown whether these variable results might be related to a different distribution of pathological α-syn in OM. Thus, we investigated whether nasal swab (NS) performed in areas with a different coverage by olfactory neuroepithelium, such as agger nasi (AN) and middle turbinate (MT), might affect the detection of pathological α-syn.
Methods: NS was performed in 66 patients with PD and 29 non-PD between September 2018 and April 2021. In 43 patients, cerebrospinal fluid (CSF) was also obtained and all samples were analyzed by RT-QuIC for α-syn.
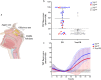
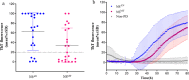
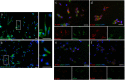
Results: In the first round, 72 OM samples were collected by NS, from AN (NSAN) or from MT (NSMT), and 35 resulted positive for α-syn RT-QuIC, including 27/32 (84%) from AN, 5/11 (45%) from MT, and 3/29 (10%) belonging to the non-PD patients. Furthermore, 23 additional PD patients underwent NS at both AN and MT, and RT-QuIC revealed α-syn positive in 18/23 (78%) NSAN samples and in 10/23 (44%) NSMT samples. Immunocytochemistry of NS preparations showed a higher representation of olfactory neural cells in NSAN compared to NSMT. We also observed α-syn and phospho-α-syn deposits in NS from PD patients but not in controls. Finally, RT-QuIC was positive in 22/24 CSF samples from PD patients (92%) and in 1/19 non-PD.
Conclusion: In PD patients, RT-QuIC sensitivity is significantly increased (from 45% to 84%) when NS is performed at AN, indicating that α-syn aggregates are preferentially detected in olfactory areas with higher concentration of olfactory neurons. Although RT-QuIC analysis of CSF showed a higher diagnostic accuracy compared to NS, due to the non-invasiveness, NS might be considered as an ancillary procedure for PD diagnosis.
Keywords: Alpha-synuclein; Cerebrospinal fluid; Olfactory mucosa; Parkinson disease; Real-time quaking-induced conversion assay.
© 2022. The Author(s).
Conflict of interest statement
All authors report no competing interests.
Figures

References
-
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:1–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

