Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations
- PMID: 35903083
- PMCID: PMC9314780
- DOI: 10.3389/fragi.2022.931331
Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations
Abstract
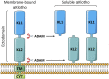
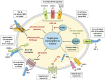
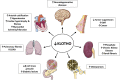
The α-Klotho protein (henceforth denoted Klotho) has antiaging properties, as first observed in mice homozygous for a hypomorphic Klotho gene (kl/kl). These mice have a shortened lifespan, stunted growth, renal disease, hyperphosphatemia, hypercalcemia, vascular calcification, cardiac hypertrophy, hypertension, pulmonary disease, cognitive impairment, multi-organ atrophy and fibrosis. Overexpression of Klotho has opposite effects, extending lifespan. In humans, Klotho levels decline with age, chronic kidney disease, diabetes, Alzheimer's disease and other conditions. Low Klotho levels correlate with an increase in the death rate from all causes. Klotho acts either as an obligate coreceptor for fibroblast growth factor 23 (FGF23), or as a soluble pleiotropic endocrine hormone (s-Klotho). It is mainly produced in the kidneys, but also in the brain, pancreas and other tissues. On renal tubular-cell membranes, it associates with FGF receptors to bind FGF23. Produced in bones, FGF23 regulates renal excretion of phosphate (phosphaturic effect) and vitamin D metabolism. Lack of Klotho or FGF23 results in hyperphosphatemia and hypervitaminosis D. With age, human renal function often deteriorates, lowering Klotho levels. This appears to promote age-related pathology. Remarkably, Klotho inhibits four pathways that have been linked to aging in various ways: Transforming growth factor β (TGF-β), insulin-like growth factor 1 (IGF-1), Wnt and NF-κB. These can induce cellular senescence, apoptosis, inflammation, immune dysfunction, fibrosis and neoplasia. Furthermore, Klotho increases cell-protective antioxidant enzymes through Nrf2 and FoxO. In accord, preclinical Klotho therapy ameliorated renal, cardiovascular, diabetes-related and neurodegenerative diseases, as well as cancer. s-Klotho protein injection was effective, but requires further investigation. Several drugs enhance circulating Klotho levels, and some cross the blood-brain barrier to potentially act in the brain. In clinical trials, increased Klotho was noted with renin-angiotensin system inhibitors (losartan, valsartan), a statin (fluvastatin), mTOR inhibitors (rapamycin, everolimus), vitamin D and pentoxifylline. In preclinical work, antidiabetic drugs (metformin, GLP-1-based, GABA, PPAR-γ agonists) also enhanced Klotho. Several traditional medicines and/or nutraceuticals increased Klotho in rodents, including astaxanthin, curcumin, ginseng, ligustilide and resveratrol. Notably, exercise and sport activity increased Klotho. This review addresses molecular, physiological and therapeutic aspects of Klotho.
Keywords: FGF23; IGF-1; NF-KappaB; TGF-beta; Wnt; aging; hyperphosphatemia; klotho.
Copyright © 2022 Prud’homme, Kurt and Wang.
Conflict of interest statement
Author QW was employed by the company Shanghai Yinuo Pharmaceutical Co., Ltd. QW holds GLP-1 related patents. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

