Role of B-Cell in the Pathogenesis of Systemic Sclerosis
- PMID: 35903091
- PMCID: PMC9315392
- DOI: 10.3389/fimmu.2022.933468
Role of B-Cell in the Pathogenesis of Systemic Sclerosis
Abstract
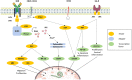
Systemic sclerosis (SSc) is a rare multisystem autoimmune disease, characterized by fibrosis, vasculopathy, and autoimmunity. Recent advances have highlighted the significant implications of B-cells in SSc. B-cells are present in affected organs, their subpopulations are disrupted, and they display an activated phenotype, and the regulatory capacities of B-cells are impaired, as illustrated by the decrease in the IL-10+ producing B-cell subpopulation or the inhibitory membrane co-receptor density. Recent multi-omics evidence highlights the role of B-cells mainly in the early stage of SSc and preferentially during severe organ involvement. This dysregulated homeostasis partly explains the synthesis of anti-endothelial cell autoantibodies (AECAs) or anti-fibroblast autoantibodies (AFAs), proinflammatory or profibrotic cytokines (interleukin-6 and transforming growth factor-β) produced by B and plasma cells. That is associated with cell-to-cell interactions with endothelial cells, fibroblasts, vascular smooth muscle cells, and other immune cells, altogether leading to cell activation and proliferation, cell resistance to apoptosis, the impairment of regulatory mechanisms, and causing fibrosis of several organs encountered in the SSc. Finally, alongside these exploratory data, treatments targeting B-cells, through their depletion by cytotoxicity (anti-CD20 monoclonal antibody), or the cytokines produced by the B-cell, or their costimulation molecules, seem interesting, probably in certain profiles of early patients with severe organic damage.
Keywords: B-cell; B-cell receptor (BCR); autoantibodies; interleukine 6 (IL-6); pathogenesis; rituximab; systemic sclerosis; transcriptomic.
Copyright © 2022 Thoreau, Chaigne and Mouthon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum (2013) 65:2737–47. doi: 10.1002/art.38098 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

