Tolerability and Effects of 2-Aminoethyl Dihydrogen Phosphate in Dogs With Mast Cell Tumors
- PMID: 35903136
- PMCID: PMC9315353
- DOI: 10.3389/fvets.2022.898077
Tolerability and Effects of 2-Aminoethyl Dihydrogen Phosphate in Dogs With Mast Cell Tumors
Abstract
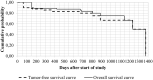
Canine mast cell tumor is a malignant neoplasm, and a gold standard treatment remains to be determined despite the proposed chemotherapies or other therapies in dogs. This study aimed to determine therapeutic, adverse effects and toxicity, tumor-free, and overall survival times of 10 dogs with surgically excised mast cell tumors evaluated by histopathological/immunohistochemistry and treated with four weekly intravenous administrations of 2-Aminoethyl Dihydrogen Phosphate (70 mg/kg) as adjuvant therapy. No adverse events were noted. Laboratory changes were limited (p < 0.05) in red blood cell, hemoglobin, and platelet counts. Mean tumor-free and overall survival were 599.1 ± 469 and 755.5 ± 423.5 days, respectively. In conclusion, 2-Aminoethyl Dihydrogen Phosphate administration was safe in dogs. However, 2-Aminoethyl Dihydrogen Phosphate was not sufficiently effective to prevent a recurrence, new tumor, or metastasis of canine mast cell tumors with poor immunohistochemical prognostic factors.
Keywords: antineoplastic phospholipids; canine; chemotherapy; immunohistochemistry; toxicity.
Copyright © 2022 Januário, Melo, Maria, Lorigados, Ambrósio, Kogika, Cogliati, Shimozako and Matera.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources