Insulin-Like Growth Factor Binding Protein (IGFBP-6) as a Novel Regulator of Inflammatory Response in Cystic Fibrosis Airway Cells
- PMID: 35903151
- PMCID: PMC9322660
- DOI: 10.3389/fmolb.2022.905468
Insulin-Like Growth Factor Binding Protein (IGFBP-6) as a Novel Regulator of Inflammatory Response in Cystic Fibrosis Airway Cells
Abstract
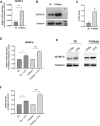
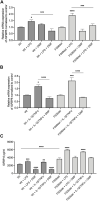
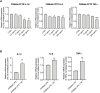
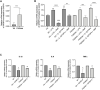
Cystic Fibrosis (CF) patients are prone to contracting bacterial lung infections with opportunistic pathogens, especially Pseudomonas aeruginosa. Prolonged P. aeruginosa infections have been linked to chronic inflammation in the CF lung, whose hallmarks are increased levels of cytokines (i.e., TNF-α, IL-1β, IL-6) and neutrophil attraction by chemokines, like IL-8. Recently, insulin-like growth factor binding protein 6 (IGFBP-6) has been shown to play a putative role in the immune system and was found at higher levels in the sera and synovial tissue of rheumatoid arthritis patients. Moreover, it has been demonstrated that IGFBP-6 has chemoattractant properties towards cells of the innate (neutrophils, monocytes) and adaptive (T cells) immunity. However, it is not known whether IGFBP-6 expression is dysregulated in airway epithelial cells under infection/inflammatory conditions. Therefore, we first measured the basal IGFBP-6 mRNA and protein levels in bronchial epithelial cells lines (Wt and F508del-CFTR CFBE), finding they both are upregulated in F508del-CFTR CFBE cells. Interestingly, LPS and IL-1β+TNFα treatments increased the IGFBP-6 mRNA level, that was reduced after treatment with an anti-inflammatory (Dimethyl Fumarate) in CFBE cell line and in patient-derived nasal epithelial cultures. Lastly, we demonstrated that IGFBP-6 reduced the level of pro-inflammatory cytokines in both CFBE and primary nasal epithelial cells, without affecting rescued CFTR expression and function. The addition of a neutralizing antibody to IGFBP-6 increased pro-inflammatory cytokines expression under challenge with LPS. Together, these data suggest that IGFBP-6 may play a direct role in the CF-associated inflammation.
Keywords: IGFBP-6; TRIKAFTA; airway epithelial cells; cystic fibrosis; cytokine; dimethyl fumarate.
Copyright © 2022 Laselva, Criscione, Allegretta, Di Gioia, Liso and Conese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abbattiscianni A. C., Favia M., Mancini M. T., Cardone R. A., Guerra L., Monterisi S., et al. (2016). Correctors of Mutant CFTR Enhance Subcortical cAMP-PKA Signaling through Modulating Ezrin Phosphorylation and Cytoskeleton Organization. J. Cell Sci. 129, 1128–1140. 10.1242/jcs.177907 - DOI - PubMed
-
- Broxmeyer H. E., Sherry B., Cooper S., Lu L., Maze R., Beckmann M. P., et al. (1993). Comparative Analysis of the Human Macrophage Inflammatory Protein Family of Cytokines (Chemokines) on Proliferation of Human Myeloid Progenitor Cells. Interacting Effects Involving Suppression, Synergistic Suppression, and Blocking of Suppression. J. Immunol. 150, 3448–3458. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

