A New Perspective on the Pathophysiology of Idiopathic Intracranial Hypertension: Role of the Glia-Neuro-Vascular Interface
- PMID: 35903170
- PMCID: PMC9315230
- DOI: 10.3389/fnmol.2022.900057
A New Perspective on the Pathophysiology of Idiopathic Intracranial Hypertension: Role of the Glia-Neuro-Vascular Interface
Abstract
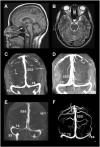
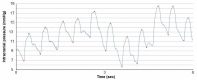
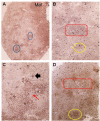
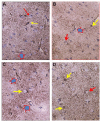
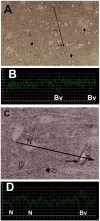
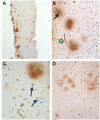
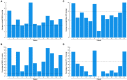
Idiopathic intracranial hypertension (IIH) is a neurological disease characterized by symptoms and signs of increased intracranial pressure (ICP) of unknown cause. Most attention has been given to the role of cerebrospinal fluid (CSF) disturbance and intracranial venous hypertension caused by sinus vein stenosis. We previously proposed that key pathophysiological processes take place within the brain at the glia-neuro-vascular interface. However, the relative importance of the proposed mechanisms in IIH disease remains unknown. Modern treatment regimens aim to reduce intracranial CSF and venous pressures, but a substantial proportion of patients experience lasting complaints. In 2010, the first author established a database for the prospective collection of information from individuals being assessed for IIH. The database incorporates clinical, imaging, physiological, and biological data, and information about treatment/outcome. This study retrieved information from the database, asking the following research questions: In IIH subjects responding to shunt surgery, what is the occurrence of signs of CSF disturbance, sinus vein stenosis, intracranial hypertension, and microscopic evidence of structural abnormalities at the glia-neuro-vascular interface? Secondarily, do semi-quantitative measures of abnormal ultrastructure at the glia-neurovascular differ between subjects with definite IIH and non-IIH (reference) subjects? The study included 13 patients with IIH who fulfilled the diagnostic criteria and who improved following shunt surgery, i.e., patients with definite IIH. Comparisons were done regarding magnetic resonance imaging (MRI) findings, pulsatile and static ICP scores, and immune-histochemistry microscopy. Among these 13 IIH subjects, 6/13 (46%) of patients presented with magnetic resonance imaging (MRI) signs of CSF disturbance (empty sella and/or distended perioptic subarachnoid spaces), 0/13 (0%) of patients with IIH had MRI signs of sinus vein stenosis, 13/13 (100%) of patients with IIH presented with abnormal preoperative pulsatile ICP [overnight mean ICP wave amplitude (MWA) above thresholds], 3/13 (23%) patients showed abnormal static ICP (overnight mean ICP above threshold), and 12/13 (92%) of patients with IIH showed abnormal structural changes at the glia-neuro-vascular interface. Comparisons of semi-quantitative structural variables between IIH and aged- and gender-matched reference (REF) subjects showed IIH abnormalities in glial cells, neurons, and capillaries. The present data suggest a key role of disease processes affecting the glia-neuro-vascular interface.
Keywords: astrocytes; benign intracranial hypertension; capillaries; idiopathic intracranial hypertension; intracranial pressure; pathophysiology; pseudotumor cerebri.
Copyright © 2022 Eide and Hansson.
Conflict of interest statement
Figure 3 is from Sensometrics analytical software, which is used for digital recording of continuous pressure (ICP, ABP, and CSF pressure) signals. PE has a financial interest in the software company, dPCom AS, which manufactures this software. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Abnormal Intracranial Pulse Pressure Amplitude Despite Normalized Static Intracranial Pressure in Idiopathic Intracranial Hypertension Refractory to Conservative Medical Therapy.Life (Basel). 2021 Jun 9;11(6):537. doi: 10.3390/life11060537. Life (Basel). 2021. PMID: 34207519 Free PMC article.
-
Comparative observational study on the clinical presentation, intracranial volume measurements, and intracranial pressure scores in patients with either Chiari malformation Type I or idiopathic intracranial hypertension.J Neurosurg. 2017 Apr;126(4):1312-1322. doi: 10.3171/2016.4.JNS152862. Epub 2016 Jun 24. J Neurosurg. 2017. PMID: 27341045
-
Do Most Patients With a Spontaneous Cerebrospinal Fluid Leak Have Idiopathic Intracranial Hypertension?J Neuroophthalmol. 2019 Dec;39(4):487-495. doi: 10.1097/WNO.0000000000000761. J Neuroophthalmol. 2019. PMID: 30747786 Free PMC article. Review.
-
The pathophysiology of chronic noncommunicating hydrocephalus: lessons from continuous intracranial pressure monitoring and ventricular infusion testing.J Neurosurg. 2018 Jul;129(1):220-233. doi: 10.3171/2017.1.JNS162813. Epub 2017 Aug 11. J Neurosurg. 2018. PMID: 28799879
-
Brain Imaging in Idiopathic Intracranial Hypertension.J Neuroophthalmol. 2015 Dec;35(4):400-11. doi: 10.1097/WNO.0000000000000303. J Neuroophthalmol. 2015. PMID: 26457687 Review.
Cited by
-
Transactivation Response DNA-Binding Protein of 43 (TDP-43) and Glial Cell Roles in Neurological Disorders.Cureus. 2022 Oct 24;14(10):e30639. doi: 10.7759/cureus.30639. eCollection 2022 Oct. Cureus. 2022. PMID: 36439561 Free PMC article. Review.
-
Progress and recognition of idiopathic intracranial hypertension: A narrative review.CNS Neurosci Ther. 2024 Aug;30(8):e14895. doi: 10.1111/cns.14895. CNS Neurosci Ther. 2024. PMID: 39097911 Free PMC article. Review.
-
A Lumped Parameter Modelling Study of Idiopathic Intracranial Hypertension Suggests the CSF Formation Rate Varies with the Capillary Transmural Pressure.Brain Sci. 2025 May 20;15(5):527. doi: 10.3390/brainsci15050527. Brain Sci. 2025. PMID: 40426698 Free PMC article.
-
Hypermobile Ehlers-Danlos syndrome and spontaneous CSF leaks: the connective tissue conundrum.Front Neurol. 2024 Jul 17;15:1452409. doi: 10.3389/fneur.2024.1452409. eCollection 2024. Front Neurol. 2024. PMID: 39087003 Free PMC article. Review.
-
Navigating the Enigma: A Comprehensive Review of Idiopathic Intracranial Hypertension.Cureus. 2024 Mar 16;16(3):e56256. doi: 10.7759/cureus.56256. eCollection 2024 Mar. Cureus. 2024. PMID: 38623134 Free PMC article. Review.
References
-
- Adderley N. J., Subramanian A., Nirantharakumar K., Yiangou A., Gokhale K. M., Mollan S. P., et al. . (2019). Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol. 76, 1088–1098. 10.1001/jamaneurol.2019.1812 - DOI - PMC - PubMed
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. (2014). Molecular Biology of the Cell. New York, NY: Garland Science.
-
- Albuquerque F. C., Dashti S. R., Hu Y. C., Newman C. B., Teleb M., McDougall C. G., et al. . (2011). Intracranial venous sinus stenting for benign intracranial hypertension: clinical indications, technique, and preliminary results. World Neurosurg. 75, 648-52; discussion 592–5. 10.1016/j.wneu.2010.11.012 - DOI - PubMed
LinkOut - more resources
Full Text Sources

