The Role of the Na+/Ca2+ Exchanger in Aberrant Intracellular Ca2+ in Cardiomyocytes of Chagas-Infected Rodents
- PMID: 35903196
- PMCID: PMC9318578
- DOI: 10.3389/fcimb.2022.890709
The Role of the Na+/Ca2+ Exchanger in Aberrant Intracellular Ca2+ in Cardiomyocytes of Chagas-Infected Rodents
Abstract
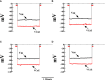
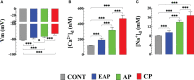
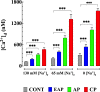
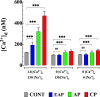
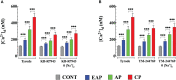
Chagas disease is produced by the parasite Trypanosoma cruzi (T. cruzi), which is the leading cause of death and morbidity in Latin America. We have shown that in patients with Chagas cardiomyopathy, there is a chronic elevation of diastolic Ca2+ concentration ([Ca2+]d), associated with deterioration to further address this issue, we explored the role Na+/Ca2+ exchanger (NCX). Experiments were carried out in noninfected C57BL/6 mice and infected with blood-derived trypomastigotes of the T. cruzi Y strain. Anesthetized mice were sacrificed and the cardiomyocytes were enzymatically dissociated. Diastolic [Ca2+] ([Ca2+]d) was measured using Ca2+ selective microelectrodes in cardiomyocytes from control mice (CONT) and cardiomyocytes from T. cruzi infected mice in the early acute phase (EAP) at 20 dpi, in the acute phase (AP) at 40 dpi, and in the chronic phase (CP) at 120 dpi. [Ca2+]d was 1.5-times higher in EAP, 2.6-times in AP, and 3.4-times in CP compared to CONT. Exploring the reverse mode activity of NCX, we replaced extracellular Na+ in equivalent amounts with N-methyl-D-glucamine. Reduction of [Na+]e to 65 mM caused an increase in [Ca2+]d of 1.7 times in cardiomyocytes from CONT mice, 2 times in EAP infected mice, 2.4 times in AP infected mice and 2.8 in CP infected mice. The Na+ free solution caused a further elevation of [Ca2+]d of 2.5 times in cardiomyocytes from CONT, 2.8 times in EAP infected mice, 3.1 times in AP infected mice, and 3.3 times in CP infected mice. Extracellular Ca2+ withdrawal reduced [Ca2+]d in both CONT and cardiomyocytes from Chagas-infected mice and prevented the increase in [Ca2+]d induced by Na+ depletion. Preincubation with 10µM KB-R7943 or in 1µM YM-244769 reduced [Ca2+]d in cardiomyocytes from infected mice, but not control mice. Furthermore, both NCX blockers prevented the increase in [Ca2+]d associated with exposure to a solution without Na+. These results suggest that Ca2+ entry through the reverse NCX mode plays a significant role in the observed [Ca2+]d dyshomeostasis in Chagas infected cardiomyocytes. Additionally, NCX inhibitors may be a viable therapeutic approach for treating patients with Chagas cardiomyopathy.
Keywords: Na/Ca exchanger; Trypanosoma cruzi (T cruzi); calcium; cardiomyopathy; chagas disease.
Copyright © 2022 Lopez, Linares, Adams and Mijares.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A novel substrate for arrhythmias in Chagas disease.PLoS Negl Trop Dis. 2021 Jun 2;15(6):e0009421. doi: 10.1371/journal.pntd.0009421. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34077437 Free PMC article.
-
Cardioprotective actions of curcumin on the pathogenic NFAT/COX-2/prostaglandin E2 pathway induced during Trypanosoma cruzi infection.Phytomedicine. 2016 Nov 15;23(12):1392-1400. doi: 10.1016/j.phymed.2016.06.017. Epub 2016 Jun 29. Phytomedicine. 2016. PMID: 27765359
-
KB-R7943 reveals possible involvement of Na(+)-Ca2+ exchanger in elevation of intracellular Ca2+ in rat carotid arterial myocytes.J Smooth Muscle Res. 2004 Feb;40(1):35-42. doi: 10.1540/jsmr.40.35. J Smooth Muscle Res. 2004. PMID: 15170076
-
Cardiac sodium transport and excitation-contraction coupling.J Mol Cell Cardiol. 2013 Aug;61:11-9. doi: 10.1016/j.yjmcc.2013.06.003. Epub 2013 Jun 14. J Mol Cell Cardiol. 2013. PMID: 23774049 Review.
-
Dual and Opposite Roles of Reactive Oxygen Species (ROS) in Chagas Disease: Beneficial on the Pathogen and Harmful on the Host.Oxid Med Cell Longev. 2020 Dec 10;2020:8867701. doi: 10.1155/2020/8867701. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33376582 Free PMC article. Review.
Cited by
-
Unraveling the role of miRNAs as biomarkers in Chagas cardiomyopathy: Insights into molecular pathophysiology.PLoS Negl Trop Dis. 2024 Feb 1;18(2):e0011865. doi: 10.1371/journal.pntd.0011865. eCollection 2024 Feb. PLoS Negl Trop Dis. 2024. PMID: 38300899 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

