Distinctive in vitro ATP Hydrolysis Activity of AtVIPP1, a Chloroplastic ESCRT-III Superfamily Protein in Arabidopsis
- PMID: 35903241
- PMCID: PMC9315428
- DOI: 10.3389/fpls.2022.949578
Distinctive in vitro ATP Hydrolysis Activity of AtVIPP1, a Chloroplastic ESCRT-III Superfamily Protein in Arabidopsis
Abstract
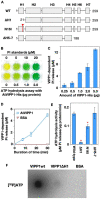
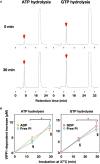
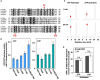
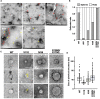
Vesicle-inducing protein in plastid 1 (VIPP1), characteristic to oxygenic photosynthetic organisms, is a membrane-remodeling factor that forms homo-oligomers and functions in thylakoid membrane formation and maintenance. The cyanobacterial VIPP1 structure revealed a monomeric folding pattern similar to that of endosomal sorting complex required for transport (ESCRT) III. Characteristic to VIPP1, however, is its own GTP and ATP hydrolytic activity without canonical domains. In this study, we found that histidine-tagged Arabidopsis VIPP1 (AtVIPP1) hydrolyzed GTP and ATP to produce GDP and ADP in vitro, respectively. Unexpectedly, the observed GTPase and ATPase activities were biochemically distinguishable, because the ATPase was optimized for alkaline conditions and dependent on Ca2+ as well as Mg2+, with a higher affinity for ATP than GTP. We found that a version of AtVIPP1 protein with a mutation in its nucleotide-binding site, as deduced from the cyanobacterial structure, retained its hydrolytic activity, suggesting that Arabidopsis and cyanobacterial VIPP1s have different properties. Negative staining particle analysis showed that AtVIPP1 formed particle or rod structures that differed from those of cyanobacteria and Chlamydomonas. These results suggested that the nucleotide hydrolytic activity and oligomer formation of VIPP1 are common in photosynthetic organisms, whereas their properties differ among species.
Keywords: ATPase; ESCRT-III superfamily; GTPase; calcium; chloroplast; photosynthesis; thylakoid membrane.
Copyright © 2022 Ohnishi, Sugimoto, Kondo, Shioya, Zhang and Sakamoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The thylakoid membrane remodeling protein VIPP1 forms bundled oligomers in tobacco chloroplasts.Plant Physiol. 2025 Apr 30;198(1):kiaf137. doi: 10.1093/plphys/kiaf137. Plant Physiol. 2025. PMID: 40198871 Free PMC article.
-
VIPP1 Involved in Chloroplast Membrane Integrity Has GTPase Activity in Vitro.Plant Physiol. 2018 May;177(1):328-338. doi: 10.1104/pp.18.00145. Epub 2018 Apr 5. Plant Physiol. 2018. PMID: 29622686 Free PMC article.
-
Structural basis for VIPP1 oligomerization and maintenance of thylakoid membrane integrity.Cell. 2021 Jul 8;184(14):3643-3659.e23. doi: 10.1016/j.cell.2021.05.011. Epub 2021 Jun 23. Cell. 2021. PMID: 34166613
-
Possible function of VIPP1 in maintaining chloroplast membranes.Biochim Biophys Acta. 2015 Sep;1847(9):831-7. doi: 10.1016/j.bbabio.2015.02.013. Epub 2015 Feb 25. Biochim Biophys Acta. 2015. PMID: 25725437 Review.
-
Conserved structures of ESCRT-III superfamily members across domains of life.Trends Biochem Sci. 2023 Nov;48(11):993-1004. doi: 10.1016/j.tibs.2023.08.009. Epub 2023 Sep 15. Trends Biochem Sci. 2023. PMID: 37718229 Review.
Cited by
-
Structural basis for Vipp1 membrane binding: from loose coats and carpets to ring and rod assemblies.Nat Struct Mol Biol. 2025 Mar;32(3):555-570. doi: 10.1038/s41594-024-01399-z. Epub 2024 Oct 8. Nat Struct Mol Biol. 2025. PMID: 39379528 Free PMC article.
-
The thylakoid membrane remodeling protein VIPP1 forms bundled oligomers in tobacco chloroplasts.Plant Physiol. 2025 Apr 30;198(1):kiaf137. doi: 10.1093/plphys/kiaf137. Plant Physiol. 2025. PMID: 40198871 Free PMC article.
-
Structural plasticity of bacterial ESCRT-III protein PspA in higher-order assemblies.Nat Struct Mol Biol. 2025 Jan;32(1):23-34. doi: 10.1038/s41594-024-01359-7. Epub 2024 Aug 16. Nat Struct Mol Biol. 2025. PMID: 39152237 Free PMC article.
-
The green ESCRTs: Newly defined roles for ESCRT proteins in plants.J Biol Chem. 2025 May;301(5):108465. doi: 10.1016/j.jbc.2025.108465. Epub 2025 Mar 27. J Biol Chem. 2025. PMID: 40157538 Free PMC article. Review.
References
-
- Akhova A. V., Tkachenko A. G. (2019). HPLC-UV method for simultaneous determination of adenosine triphosphate and Its metabolites in Mycobacterium smegmatis. Acta Chromatogr. 31 45–48. 10.1556/1326.2017.00344 - DOI
-
- Berges J. A., Montagnes D. J. S., Hurd C. L., Harrison P. J. (1994). Fitting ecological and physiological data to rectangular hyperbolae - a comparison of methods using Monte Carlo simulations. Mar. Ecol. Prog. Ser. 114 175–183. 10.3354/meps114175 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

