Noninvasive Evaluation of EGFR Expression of Digestive Tumors Using 99mTc-MAG3-Cet-F(ab')2-Based SPECT/CT Imaging
- PMID: 35903247
- PMCID: PMC9281432
- DOI: 10.1155/2022/3748315
Noninvasive Evaluation of EGFR Expression of Digestive Tumors Using 99mTc-MAG3-Cet-F(ab')2-Based SPECT/CT Imaging
Abstract
Purpose: This study is aimed at investigating the feasibility of cetuximab (Cet) F(ab')2 fragment- (Cet-F(ab')2-) based single photon emission tomography/computed tomography (SPECT/CT) for assessing the epidermal growth factor receptor (EGFR) expression in digestive tumor mouse models.
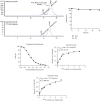
Methods: Cet-F(ab')2 was synthesized using immunoglobulin G-degrading enzyme of Streptococcus pyogenes (IdeS) protease and purified with protein A beads. The product and its in vitro stability in normal saline and 1% bovine serum albumin were analyzed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The EGFR expression in the human colon tumor cell line HT29 and the human stomach tumor cell line MGC803 were verified using western blotting and immunocytochemistry. Cet-F(ab')2 was conjugated with 5(6)-carboxytetramethylrhodamine succinimidyl ester to demonstrate its binding ability to the MGC803 and HT29 cells. Cet-F(ab')2 was conjugated with NHS-MAG3 for 99mTc radiolabeling. The best imaging time was determined using a biodistribution assay at 1, 4, 16, and 24 h after injection of the 99mTc-MAG3-Cet-F(ab')2 tracer. Furthermore, 99mTc-MAG3-Cet-F(ab')2 SPECT/CT was performed on MGC803 and HT29 tumor-bearing nude mice.
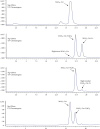
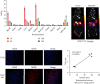
Results: HT29 cells had low EGFR expression while MGC803 cell exhibited the high EGFR expression. Cet-F(ab')2 and intact cetuximab showed similar high binding ability to MGC803 cells but not to HT29 cells. Cet-F(ab')2 and 99mTc-MAG3-Cet-F(ab')2 showed excellent in vitro stability. The biodistribution assay showed that the target to nontarget ratio was the highest at 16 h (17.29 ± 5.72, n = 4) after tracer injection. The 99mTc-MAG3-Cet-F(ab')2-based SPECT/CT imaging revealed rapid and sustained tracer uptake in MGC803 tumors rather than in HT29 tumors with high image contrast, which was consistent with the results in vitro.
Conclusion: SPECT/CT imaging using 99mTc-MAG3-Cet-F(ab')2 enables the evaluation of the EGFR expression in murine EGFR-positive tumors, indicating the potential utility for noninvasive evaluation of the EGFR expression in tumors.
Copyright © 2022 Dai Shi et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding this article.
Figures



Similar articles
-
Preclinical Evaluation of 99mTc-MAG3-5-Fab Targeting TREM2 in Lung Cancer Mouse Models: A Comparison with 99mTc-MAG3-5-F(ab')2.Mol Pharm. 2024 Jan 1;21(1):303-312. doi: 10.1021/acs.molpharmaceut.3c00870. Epub 2023 Dec 18. Mol Pharm. 2024. PMID: 38109713
-
Imaging of epidermal growth factor receptor expression in head and neck cancer with SPECT/CT and 111In-labeled cetuximab-F(ab')2.J Nucl Med. 2013 Dec;54(12):2118-24. doi: 10.2967/jnumed.113.123612. Epub 2013 Oct 17. J Nucl Med. 2013. PMID: 24136932
-
Early response monitoring with 18F-FDG PET and cetuximab-F(ab')2-SPECT after radiotherapy of human head and neck squamous cell carcinomas in a mouse model.J Nucl Med. 2014 Oct;55(10):1665-70. doi: 10.2967/jnumed.114.141762. Epub 2014 Sep 18. J Nucl Med. 2014. PMID: 25236350
-
111In/86Y-Labeled F(ab')2 fragment of panitumumab, a fully human monoclonal antibody directed against the extracellular domain III of the epidermal growth factor receptor.2012 Apr 30 [updated 2012 Jun 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Apr 30 [updated 2012 Jun 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22741182 Free Books & Documents. Review.
-
99mTc-Mercaptoacetyl-Glu-Glu-Glu-Affibody ZHER2:342.2008 Mar 16 [updated 2008 Apr 7]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Mar 16 [updated 2008 Apr 7]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641930 Free Books & Documents. Review.
Cited by
-
Noninvasive evaluation of PD-L1 expression in non-small cell lung cancer by immunoPET imaging using an acylating agent-modified antibody fragment.Eur J Nucl Med Mol Imaging. 2023 May;50(6):1585-1596. doi: 10.1007/s00259-023-06130-6. Epub 2023 Feb 10. Eur J Nucl Med Mol Imaging. 2023. PMID: 36759371
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

