Energy Metabolism and Lipidome Are Highly Regulated during Osteogenic Differentiation of Dental Follicle Cells
- PMID: 35903407
- PMCID: PMC9315453
- DOI: 10.1155/2022/3674931
Energy Metabolism and Lipidome Are Highly Regulated during Osteogenic Differentiation of Dental Follicle Cells
Abstract
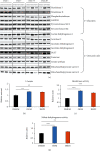
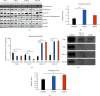
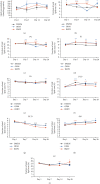
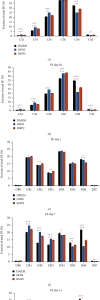
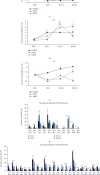
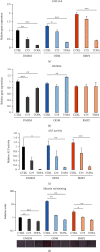
Dental follicle cells (DFCs) are stem/progenitor cells of the periodontium and give rise to alveolar osteoblasts. However, understanding of the molecular mechanisms of osteogenic differentiation, which is required for cell-based therapies, is delimited. This study is aimed at analyzing the energy metabolism during the osteogenic differentiation of DFCs. Human DFCs were cultured, and osteogenic differentiation was induced by either dexamethasone or bone morphogenetic protein 2 (BMP2). Previous microarray data were reanalyzed to examine pathways that are regulated after osteogenic induction. Expression and activity of metabolic markers were evaluated by western blot analysis and specific assays, relative amount of mitochondrial DNA was measured by real-time quantitative polymerase chain reaction, the oxidative state of cells was determined by a glutathione assay, and the lipidome of cells was analyzed via mass spectrometry (MS). Moreover, osteogenic markers were analyzed after the inhibition of fatty acid synthesis by 5-(tetradecyloxy)-2-furoic acid or C75. Pathway enrichment analysis of microarray data revealed that carbon metabolism was amongst the top regulated pathways after osteogenic induction in DFCs. Further analysis showed that enzymes involved in glycolysis, citric acid cycle, mitochondrial activity, and lipid metabolism are differentially expressed during differentiation, with most markers upregulated and more markedly after induction with dexamethasone compared to BMP2. Moreover, the cellular state was more oxidized, and mitochondrial DNA was distinctly upregulated during the second half of differentiation. Besides, MS of the lipidome revealed higher lipid concentrations after osteogenic induction, with a preference for species with lower numbers of C-atoms and double bonds, which indicates a de novo synthesis of lipids. Concordantly, inhibition of fatty acid synthesis impeded the osteogenic differentiation of DFCs. This study demonstrates that energy metabolism is highly regulated during osteogenic differentiation of DFCs including changes in the lipidome suggesting enhanced de novo synthesis of lipids, which are required for the differentiation process.
Copyright © 2022 Oliver Pieles et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures

Similar articles
-
Evaluation of Current Studies to Elucidate Processes in Dental Follicle Cells Driving Osteogenic Differentiation.Biomedicines. 2023 Oct 13;11(10):2787. doi: 10.3390/biomedicines11102787. Biomedicines. 2023. PMID: 37893160 Free PMC article. Review.
-
Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB.Stem Cell Res Ther. 2021 Apr 14;12(1):242. doi: 10.1186/s13287-021-02313-w. Stem Cell Res Ther. 2021. PMID: 33853677 Free PMC article.
-
NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells.Biochem Biophys Res Commun. 2014 Jan 10;443(2):500-4. doi: 10.1016/j.bbrc.2013.11.120. Epub 2013 Dec 7. Biochem Biophys Res Commun. 2014. PMID: 24321094
-
The hedgehog-signaling pathway is repressed during the osteogenic differentiation of dental follicle cells.Mol Cell Biochem. 2017 Apr;428(1-2):79-86. doi: 10.1007/s11010-016-2918-4. Epub 2017 Jan 23. Mol Cell Biochem. 2017. PMID: 28116542
-
The dexamethasone induced osteogenic differentiation of dental follicle cells.Histol Histopathol. 2017 Dec;32(12):1223-1229. doi: 10.14670/HH-11-907. Epub 2017 May 30. Histol Histopathol. 2017. PMID: 28555715 Review.
Cited by
-
Mitochondria in Multi-Directional Differentiation of Dental-Derived Mesenchymal Stem Cells.Biomolecules. 2023 Dec 21;14(1):12. doi: 10.3390/biom14010012. Biomolecules. 2023. PMID: 38275753 Free PMC article. Review.
-
Mesenchymal stem cells-derived extracellular vesicles protect against oxidative stress-induced xenogeneic biological root injury via adaptive regulation of the PI3K/Akt/NRF2 pathway.J Nanobiotechnology. 2023 Dec 4;21(1):466. doi: 10.1186/s12951-023-02214-5. J Nanobiotechnology. 2023. PMID: 38049845 Free PMC article.
-
Evaluation of Current Studies to Elucidate Processes in Dental Follicle Cells Driving Osteogenic Differentiation.Biomedicines. 2023 Oct 13;11(10):2787. doi: 10.3390/biomedicines11102787. Biomedicines. 2023. PMID: 37893160 Free PMC article. Review.
-
Matricellular Protein SMOC2 Potentiates BMP9-Induced Osteogenic Differentiation in Mesenchymal Stem Cells through the Enhancement of FAK/PI3K/AKT Signaling.Stem Cells Int. 2023 Jan 16;2023:5915988. doi: 10.1155/2023/5915988. eCollection 2023. Stem Cells Int. 2023. PMID: 36698376 Free PMC article.
References
-
- Zavan B., Bressan E., editors. Dental Stem Cells: Regenerative Potential . Cham: Springer International Publishing; 2016. - DOI
LinkOut - more resources
Full Text Sources

