Omega-3 Polyunsaturated Fatty Acids Supplementation Alleviate Anxiety Rather Than Depressive Symptoms Among First-Diagnosed, Drug-Naïve Major Depressive Disorder Patients: A Randomized Clinical Trial
- PMID: 35903448
- PMCID: PMC9315396
- DOI: 10.3389/fnut.2022.876152
Omega-3 Polyunsaturated Fatty Acids Supplementation Alleviate Anxiety Rather Than Depressive Symptoms Among First-Diagnosed, Drug-Naïve Major Depressive Disorder Patients: A Randomized Clinical Trial
Abstract
Background: Omega-3 polyunsaturated fatty acids (n-3 PUFAs) augmentation of antidepressants has shown great potential in the prevention and treatment of major depressive disorders (MDD).
Objective: To investigate the effect of n-3 PUFAs plus venlafaxine in patients with first-diagnosed, drug-naïve depression.
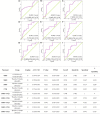
Method: A total of 72 outpatients with first-diagnosed depression were recruited. The daily dose of 2.4 g/day n-3 PUFAs or placebo plus venlafaxine was used for over 12 weeks. The outcomes were assessed by the Hamilton depression scale (HAMD), Hamilton anxiety scale (HAMA), Beck depression inventory (BDI), and Self-rating anxiety scale (SAS).
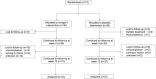
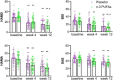
Results: Both groups exhibited improvement on clinical characteristics at week 4 and week 12 compared with baseline. The rate of responders for anxiety in n-3 PUFAs group (44.44%) was significantly higher than that in placebo group (21.21%) at week 4 (χ2 = 4.182, p = 0.041), while week 12 did not show a difference (χ2 = 0.900, p = 0.343). The rate of responders for depression at both week 4 (χ2 = 0.261, p = 0.609) and week 12 (χ2 = 1.443, p = 0.230) showed no significant difference between two groups. Further analysis found that Childhood Trauma Questionnaire (CTQ) had positive correlation with HAMA (r = 0.301, p = 0.012), SAS (r = 0.246, p = 0.015), HAMD (r = 0.252, p = 0.038) and BDI (r = 0.233, p = 0.022) with Pearson correlation analysis. Social Support Rating Scale (SSRS) had negative correlation with SAS (r = -0.244, p = 0.015) and BDI (r = -0.365, p = 0.000).
Conclusion: This trial found that n-3 PUFAs supplementation in favor of venlafaxine alleviated the anxiety symptoms rather than depressive symptoms at the early stage of treatment (4 weeks) for first-diagnosed, drug-naïve depressed patients. However, the advantage disappeared in long-term treatment. Furthermore, childhood abuse and social support are closely related to the clinical and biological characteristics of depression. Both childhood trauma and lack of social support might be predictors of poor prognosis in depression.
Clinical trial registration: [clinicaltrials.gov], identifier [NCT03295708].
Keywords: antidepressant; childhood abuse; major depressive disorders; nutrient intervention; omega-3 polyunsaturated fatty acids; social support.
Copyright © 2022 Yang, Wang, Jin, Cao, Wu, Guo, Chen, Tang and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

