Preventive Effects of Long-Term Intake of Plant Oils With Different Linoleic Acid/Alpha-Linolenic Acid Ratios on Acute Colitis Mouse Model
- PMID: 35903457
- PMCID: PMC9315388
- DOI: 10.3389/fnut.2022.788775
Preventive Effects of Long-Term Intake of Plant Oils With Different Linoleic Acid/Alpha-Linolenic Acid Ratios on Acute Colitis Mouse Model
Abstract
Objective: To investigate the preventive effects of plant oils with different linoleic acid/alpha-linolenic acid (LA/ALA) ratios against colitis symptoms, and dysbiosis of gut microbiota in acute colitis mouse model.
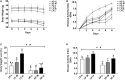
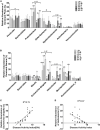
Methods: Sixty male C57BL/6 mice were assigned into six groups (n = 10): three groups were fed low-fat diets with low, medium, and high LA/ALA ratios; and three groups were fed with high-fat diets with low, medium, and high LA/ALA ratios. After 3 months of diet, the mice were exposed to dextran sodium sulfate solution to induce acute colitis. The severity of colitis was estimated by disease activity index (DAI) and histopathological examination. 16S rRNA gene sequencing was used for the analysis of gut microbiota.
Results: Plant oils with a lower LA/ALA ratio showed higher alleviating effects on the symptoms of colitis, which were accompanied by the better prebiotic characteristics manifested as effectively inhibiting the abnormal expansion of phylum Proteobacteria and genus Escherichia-Shigella in the gut microbiota of colitis mouse models.
Conclusion: A potential IBD prevention strategy of reducing the LA/ALA ratio in the daily consumed plant oils was proposed in this study. Furthermore, based on the optimized LA/ALA ratio, this preventive effect might not be weakened by the high intake of plant oils.
Keywords: 16S rRNA gene sequencing; alpha-linolenic acid; colitis mouse model; gut microbiota; inflammation; linoleic acid.
Copyright © 2022 Wang, Yue, Zhang, Wan, Ji and Geng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with α-linolenic acid in a rat model of inflammatory bowel disease.Br J Nutr. 2012 Nov 14;108(9):1612-22. doi: 10.1017/S0007114511007197. Epub 2012 Jan 16. Br J Nutr. 2012. PMID: 22243775
-
Dextran Sulfate Sodium Salt-Induced Colitis Aggravates Gut Microbiota Dysbiosis and Liver Injury in Mice With Non-alcoholic Steatohepatitis.Front Microbiol. 2021 Nov 2;12:756299. doi: 10.3389/fmicb.2021.756299. eCollection 2021. Front Microbiol. 2021. PMID: 34795650 Free PMC article.
-
Endogenous production of n-3 long-chain PUFA from first feeding and the influence of dietary linoleic acid and the α-linolenic:linoleic ratio in Atlantic salmon (Salmo salar).Br J Nutr. 2019 Nov 28;122(10):1091-1102. doi: 10.1017/S0007114519001946. Epub 2019 Aug 14. Br J Nutr. 2019. PMID: 31409428
-
Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice.Sci Rep. 2018 Oct 8;8(1):14916. doi: 10.1038/s41598-018-33092-9. Sci Rep. 2018. PMID: 30297695 Free PMC article.
-
Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration?Nutrients. 2019 Oct 4;11(10):2365. doi: 10.3390/nu11102365. Nutrients. 2019. PMID: 31590264 Free PMC article. Review.
Cited by
-
Influence of α-Linolenic Acid on the Intestinal Barrier Integrity and Intestinal Antioxidant Status in Broilers.Food Sci Nutr. 2025 May 28;13(6):e70271. doi: 10.1002/fsn3.70271. eCollection 2025 Jun. Food Sci Nutr. 2025. PMID: 40438093 Free PMC article.
References
LinkOut - more resources
Full Text Sources

