Edaravone Modulates Neuronal GPX4/ACSL4/5-LOX to Promote Recovery After Spinal Cord Injury
- PMID: 35903552
- PMCID: PMC9318422
- DOI: 10.3389/fcell.2022.849854
Edaravone Modulates Neuronal GPX4/ACSL4/5-LOX to Promote Recovery After Spinal Cord Injury
Abstract
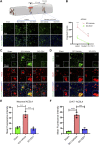
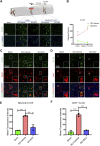
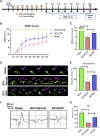
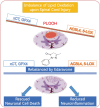
The FDA-approved drug edaravone has a neuroprotective effect on spinal cord injury (SCI) and many other central nervous system diseases. However, its molecular mechanism remains unclear. Since edaravone is a lipid peroxidation scavenger, we hypothesize that edaravone exerts its neuroprotective effect by inhibiting ferroptosis in SCI. Edaravone treatment after SCI upregulates glutathione peroxidase 4 (GPX4) and system Xc-light chain (xCT), which are anti-ferroptosis proteins. It downregulates pro-ferroptosis proteins Acyl-CoA synthetase long-chain family member 4 (ACSL4) and 5-lipoxygenase (5-LOX). The most significant changes in edaravone treatment occur in the acute phase, two days post injury. Edaravone modulates neuronal GPX4/ACSL4/5-LOX in the spinal segment below the lesion, which is critical for maintaining locomotion. Moreover, the GPX4/ACSL4/5-LOX in motor neuron is also modulated by edaravone in the spinal cord. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. The cytokine array revealed that edaravone upregulated some anti-inflammatory cytokines such as IL-10, IL-13, and adiponectin. Edaravone reduced microgliosis and astrogliosis, indicating reduced neuroinflammation. Edaravone has a long-term effect on neuronal survival, spinal cord tissue sparing, and motor function recovery. In summary, we revealed a novel mechanism of edaravone in inhibiting neuronal ferroptosis in SCI. This mechanism may be generalizable to other neurological diseases.
Keywords: edaravone; ferroptosis; neuroinflammation; neuroprotection; spinal cord injury.
Copyright © 2022 Pang, Liu, Wang, Shi, Ma, Zhang, Zhou, Zhao, Zhang, Fan, Hao, Li, Zhao, Zhang, Zhou, Kong, Feng and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bracken M. B., Shepard M. J., Holford T. R., Leo-Summers L., Aldrich E. F., Fazl M., et al. (1997). Administration of Methylprednisolone for 24 or 48 hours or Tirilazad Mesylate for 48 hours in the Treatment of Acute Spinal Cord Injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277 (20), 1597–1604. 10.1001/jama.1997.03540440031029 - DOI - PubMed
LinkOut - more resources
Full Text Sources

