Isolation of the hemeoxygenase-1 inducer from rice-derived peptide
- PMID: 35903607
- PMCID: PMC9309089
- DOI: 10.3164/jcbn.21-125
Isolation of the hemeoxygenase-1 inducer from rice-derived peptide
Abstract
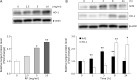
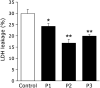
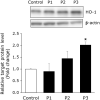
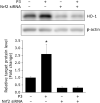
Bioactive peptides with various health benefits have been reported from rice protein hydrolysates. We previously showed that rice-derived peptides (RP) increased intracellular glutathione levels and induced the expression of γ-glutamylcysteine synthetase, which is regulated by nuclear transcription factor-erythroid 2-related factor 2 (Nrf2). Heme oxygenase-1 (HO-1) is an important Nrf2 downstream antioxidant enzyme that protects against oxidative stress. This study aimed to investigate the protective effects of RP on hydrogen peroxide (H2O2)-induced oxidative stress in human hepatoblastoma cell line HepG2 and identified HO-1 induced peptides from RP. Pretreatment of cells with RP reduced the cytotoxicity caused by H2O2 in a dose-dependent manner. Moreover, RP induced HO-1 expression in a concentration- and time-dependent manner. Next, we attempted to isolate the HO-1 inducer from RP by bioactivity-guided fractionation. Purification of the active peptides using a Sep-Pak C18 cartridge and reversed-phase HPLC, followed by sequence analysis by mass spectrometry, led to the identification of the three peptides. These peptides effectively reduced H2O2-induced oxidative stress. Among them, only P3 (peptide sequence: RSAVLLSH) increased HO-1 protein expression. Additionally, the knockdown of Nrf2 suppressed the induction of HO-1 expression by P3. Our results indicated that P3 identified from RP induced HO-1 by activating the Nrf2 signaling pathway.
Keywords: cytoprotection; heme oxygenase-1; nuclear transcription factor-erythroid 2-related factor 2; reverse-phase high-performance liquid chromatography; rice-derived peptides.
Copyright © 2022 JCBN.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Babel RA, Dandekar MP. A review on cellular and molecular mechanisms linked to the development of diabetes complications. Curr Diabetes Rev 2021; 17: 457–473. - PubMed
-
- Cross CE, Halliwell B, Borish ET, et al. Oxygen radicals and human disease. Ann Intern Med 1987; 107: 526–545. - PubMed
-
- Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun 1993; 19: 141–158. - PubMed
-
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Medicine 2018; 54: 287–293.
-
- Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 2016; 8: 33–42.
LinkOut - more resources
Full Text Sources
Research Materials

