Piezo channels contribute to the regulation of myelination in Schwann cells
- PMID: 35903933
- PMCID: PMC10638658
- DOI: 10.1002/glia.24251
Piezo channels contribute to the regulation of myelination in Schwann cells
Abstract
Peripheral nerves and Schwann cells have to sustain constant mechanical constraints, caused by developmental growth as well as stretches associated with movements of the limbs and mechanical compressions from daily activities. In Schwann cells, signaling molecules sensitive to stiffness or stretch of the extracellular matrix, such as YAP/TAZ, have been shown to be critical for Schwann cell development and peripheral nerve regeneration. YAP/TAZ have also been suggested to contribute to tumorigenesis, neuropathic pain, and inherited disorders. Yet, the role of mechanosensitive ion channels in myelinating Schwann cells is vastly unexplored. Here we comprehensively assessed the expression of mechanosensitive ion channels in Schwann cells and identified that PIEZO1 and PIEZO2 are among the most abundant mechanosensitive ion channels expressed by Schwann cells. Using classic genetic ablation studies, we show that PIEZO1 is a transient inhibitor of radial and longitudinal myelination in Schwann cells. Contrastingly, we show that PIEZO2 may be required for myelin formation, as the absence of PIEZO2 in Schwann cells delays myelin formation. We found an epistatic relationship between PIEZO1 and PIEZO2, at both the morphological and molecular levels. Finally, we show that PIEZO1 channels affect the regulation of YAP/TAZ activation in Schwann cells. Overall, we present here the first demonstration that PIEZO1 and PIEZO2 contribute to mechanosensation in Schwann cells as well myelin development in the peripheral nervous system.
Keywords: Fam38a; Fam38b; PIEZO1; PIEZO2; Schwann cell; myelin.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Figures



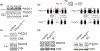



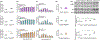


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

