N-Heterocyclic Carbene (NHC) Silver Complexes as Versatile Chemotherapeutic Agents Targeting Human Topoisomerases and Actin
- PMID: 35904129
- PMCID: PMC9804882
- DOI: 10.1002/cmdc.202200345
N-Heterocyclic Carbene (NHC) Silver Complexes as Versatile Chemotherapeutic Agents Targeting Human Topoisomerases and Actin
Abstract
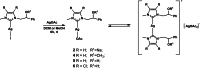
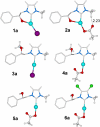
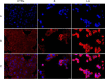
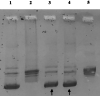
In recent years, the number of people suffering from cancer has risen rapidly and the World Health Organization and U.S. and European governments have identified this pathology as a priority issue. It is known that most bioactive anticancer molecules do not target a single protein but exert pleiotropic effects, simultaneously affecting multiple pathways. In our study, we designed and synthesized a new series of silver N-heterocyclic carbene (NHC) complexes [(NHC)2 Ag]+ [AgX2 ]- (X=iodide or acetate). The new complexes were active against two human breast cancer cell lines, MCF-7 and MDA-MB-231. These compounds showed multiple target actions as anticancer, by inhibiting in vitro the activity of the human topoisomerases I and II and interfering with the cytoskeleton dynamic, as also confirmed by in silico studies. Moreover, the antimicrobial activity of these silver complexes was studied against Gram-positive/negative bacteria. These dual properties provide a two-tiered approach, making these compounds of interest to be further deepened for the development of new chemotherapeutic agents.
Keywords: actin; breast cancer; docking studies; human topoisomerases; silver N-heterocyclic carbine complexes.
© 2022 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Coinage Metal N-Heterocyclic Carbene Complexes: Recent Synthetic Strategies and Medicinal Applications.ChemMedChem. 2021 May 6;16(9):1360-1390. doi: 10.1002/cmdc.202000836. Epub 2021 Feb 23. ChemMedChem. 2021. PMID: 33277791 Review.
-
Cytotoxicity, Cell Line Selectivity and Proapoptotic Activity of New Anticancer Agents Derived From N,N'-Functionalised Benzimidazolium Salts and Their Silver(I)-N-Heterocyclic Carbene Complexes.Drug Dev Res. 2025 May;86(3):e70100. doi: 10.1002/ddr.70100. Drug Dev Res. 2025. PMID: 40342063
-
Novel benzyl-substituted N-heterocyclic carbene-silver acetate complexes: synthesis, cytotoxicity and antibacterial studies.Metallomics. 2011 Jan;3(1):74-88. doi: 10.1039/c0mt00034e. Epub 2010 Dec 6. Metallomics. 2011. PMID: 21135954
-
Structure-Activity Relationships in NHC-Silver Complexes as Antimicrobial Agents.Molecules. 2023 May 30;28(11):4435. doi: 10.3390/molecules28114435. Molecules. 2023. PMID: 37298911 Free PMC article. Review.
-
Silver N-Heterocyclic Biscarbene Complexes: Potent Inhibitors of Thioredoxin Reductase with Anticancer Activity in Vitro and in Vivo.Chem Asian J. 2025 Apr 3;20(7):e202401672. doi: 10.1002/asia.202401672. Epub 2025 Feb 6. Chem Asian J. 2025. PMID: 39824765
Cited by
-
Silver Organometallics that are Highly Potent Thioredoxin and Glutathione Reductase Inhibitors: Exploring the Correlations of Solution Chemistry with the Strong Antibacterial Effects.ACS Infect Dis. 2024 May 10;10(5):1753-1766. doi: 10.1021/acsinfecdis.4c00104. Epub 2024 Apr 12. ACS Infect Dis. 2024. PMID: 38606463 Free PMC article.
-
Biological Activities of Ruthenium NHC Complexes: An Update.Antibiotics (Basel). 2023 Feb 9;12(2):365. doi: 10.3390/antibiotics12020365. Antibiotics (Basel). 2023. PMID: 36830276 Free PMC article. Review.
-
Novel Au(I)- and Ag(I)-NHC Complexes with N-Boc-Protected Proline as Potential Candidates for Neurodegenerative Disorders.Int J Mol Sci. 2025 Jun 25;26(13):6116. doi: 10.3390/ijms26136116. Int J Mol Sci. 2025. PMID: 40649893 Free PMC article.
-
Antibacterial and Anti-Influenza Activities of N-Heterocyclic Carbene-Gold Complexes.Pharmaceuticals (Basel). 2024 Dec 12;17(12):1680. doi: 10.3390/ph17121680. Pharmaceuticals (Basel). 2024. PMID: 39770522 Free PMC article.
-
Bottom-Up Strategy to Forecast the Drug Location and Release Kinetics in Antitumoral Electrospun Drug Delivery Systems.Int J Mol Sci. 2023 Jan 12;24(2):1507. doi: 10.3390/ijms24021507. Int J Mol Sci. 2023. PMID: 36675021 Free PMC article.
References
-
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., Ca-Cancer J. Clin. 2021, 71, 7–33. - PubMed
-
- None
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A., Ca-Cancer J. Clin. 2018, 68, 394–424; - PubMed
-
- Scrivano L., Parisi O. I., Iacopetta D., Ruffo M., Ceramella J., Sinicropi M. S., Puoci F., Polym. Adv. Technol. 2019, 30, 743–748.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

