Longitudinal comparison of the self-entry Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-RSE) and Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS) as outcome measures in people with amyotrophic lateral sclerosis
- PMID: 35904151
- PMCID: PMC9804793
- DOI: 10.1002/mus.27691
Longitudinal comparison of the self-entry Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-RSE) and Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS) as outcome measures in people with amyotrophic lateral sclerosis
Abstract
Introduction/aims: Improved functional outcome measures in amyotrophic lateral sclerosis (ALS) would aid ALS trial design and help hasten drug discovery. We evaluate the longitudinal performance of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS) compared to the Amyotrophic Lateral Sclerosis Functional Rating Scale Revised for Self-Entry (ALSFRS-RSE) as patient reported outcomes of functional status in people with ALS.
Methods: Participants completed the ROADS and the ALSFRS-RSE questionnaires at baseline, 3-, 6-, and 12- mo using Research Electronic Data Capture as part of a prospective, longitudinal, remote, online survey study of fatigue in ALS from 9/2020 to 12/2021. The scales were compared cross-sectionally (at baseline) and longitudinally. Correlation coefficients, coefficients of variation, and descriptive statistics were assessed.
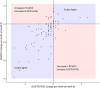
Results: A total of 182 adults with ALS consented to the study. This volunteer sample was comprised of predominantly White, non-Hispanic, non-smoking participants. Consented participant survey completion was approximately 90% at baseline and greater than 40% at 12 mo. The ALSFRS-RSE and the ROADS had high, significant agreement at 3 and 6 mo by Cohen's kappa ≥71% (p < 0.001); the number of functional increases or plateaus on the two scales were not significantly different; and the coefficient of variation of functional decline was similar at the 6-month mark, though higher for the ROADS at 3 mo and lower at 12 mo.
Discussion: Although the ROADS performed similarly to the ALSFRS-RSE in an observational cohort, it has psychometric advantages, such as Rasch-modeling and unidimensionality. It merits further investigation as a patient reported outcome of overall disability and efficacy outcome measure in ALS trials.
Keywords: ALSFRS-R; ALSFRS-RSE; ROADS; amyotrophic lateral sclerosis; outcome measure.
© 2022 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
SAJ has received research support from the ALS Association. JDB has received research support from Biogen, MT Pharma of America, MT Pharma Holdings of America, Transposon Therapeutics, Alexion, Rapa Therapeutics, ALS Association, Muscular Dystrophy Association, ALS One, Tambourine, ALS Finding a Cure. He has been a paid member of an advisory panel for Regeneron, Biogen, Clene Nanomedicine, MT Pharma of America, MT Pharma Holdings of America, Janssen, RRT. He received an honorarium for an educational event for Projects in Knowledge. He has unpaid roles on the advisory boards for the non‐profits Everything ALS and ALS One. The remaining authors have no conflicts of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

