Mimicking chronic glaucoma over 6 months with a single intracameral injection of dexamethasone/fibronectin-loaded PLGA microspheres
- PMID: 35904152
- PMCID: PMC9341346
- DOI: 10.1080/10717544.2022.2096712
Mimicking chronic glaucoma over 6 months with a single intracameral injection of dexamethasone/fibronectin-loaded PLGA microspheres
Abstract
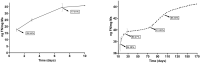
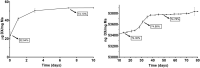
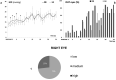
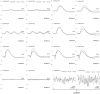
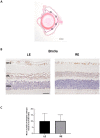
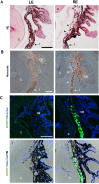
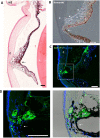
To create a chronic glaucoma animal model by a single intracameral injection of biodegradable poly lactic-co-glycolic acid (PLGA) microspheres (Ms) co-loaded with dexamethasone and fibronectin (MsDexaFibro). MsDexaFibro were prepared by a water-in-oil-in-water emulsion method including dexamethasone in the organic phase and fibronectin in the inner aqueous phase. To create the chronic glaucoma model, an interventionist and longitudinal animal study was performed using forty-five Long Evans rats (4-week-old). Rats received a single intracameral injection of MsDexafibro suspension (10%w/v) in the right eye. Ophthalmological parameters such as clinical signs, intraocular pressure (IOP), neuro-retinal functionality by electroretinography (ERG), retinal structural analysis by optical coherence tomography (OCT), and histology were evaluated up to six months. According to the results obtained, the model proposed was able to induce IOP increasing in both eyes over the study, higher in the injected eyes up to 6 weeks (p < 0.05), while preserving the ocular surface. OCT quantified progressive neuro-retinal degeneration (mainly in the retinal nerve fiber layer) in both eyes but higher in the injected eye. Ganglion cell functionality decreased in injected eyes, thus smaller amplitudes in PhNR were detected by ERG. In conclusion, a new chronic glaucoma animal model was created by a single injection of MsDexaFibro very similar to open-angle glaucoma occurring in humans. This model would impact in different fields such as ophthalmology, allowing long period of study of this pathology; pharmacology, evaluating the neuroprotective activity of active compounds; and pharmaceutical technology, allowing the correct evaluation of the efficacy of long-term sustained ocular drug delivery systems.
Keywords: Glaucoma animal model; PLGA microspheres; intraocular pressure; multiloaded; neurodegeneration.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
-
- Anderson DR. (2003). Collaborative normal tension glaucoma study. Curr Opin Ophthalmol 14:86–90. - PubMed
-
- Arranz-Romera A, Esteban-Pérez S, Garcia-Herranz D, et al. (2019). Combination therapy and co-delivery strategies to optimize treatment of posterior segment neurodegenerative diseases. Drug Discov Today 24:1644–53. - PubMed
-
- Arranz-Romera A, Hernandez M, Checa-Casalengua P, et al. (2021). A safe GDNF and GDNF/BDNF controlled delivery system improves migration in human retinal pigment epithelial cells and survival in retinal ganglion cells: potential usefulness in degenerative retinal pathologies. Pharmaceuticals 14:50. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
