The SPTLC1 p.S331 mutation bridges sensory neuropathy and motor neuron disease and has implications for treatment
- PMID: 35904184
- PMCID: PMC9804203
- DOI: 10.1111/nan.12842
The SPTLC1 p.S331 mutation bridges sensory neuropathy and motor neuron disease and has implications for treatment
Abstract
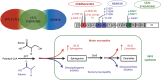
Aims: SPTLC1-related disorder is a late onset sensory-autonomic neuropathy associated with perturbed sphingolipid homeostasis which can be improved by supplementation with the serine palmitoyl-CoA transferase (SPT) substrate, l-serine. Recently, a juvenile form of motor neuron disease has been linked to SPTLC1 variants. Variants affecting the p.S331 residue of SPTLC1 cause a distinct phenotype, whose pathogenic basis has not been established. This study aims to define the neuropathological and biochemical consequences of the SPTLC1 p.S331 variant, and test response to l-serine in this specific genotype.
Methods: We report clinical and neurophysiological characterisation of two unrelated children carrying distinct p.S331 SPTLC1 variants. The neuropathology was investigated by analysis of sural nerve and skin innervation. To clarify the biochemical consequences of the p.S331 variant, we performed sphingolipidomic profiling of serum and skin fibroblasts. We also tested the effect of l-serine supplementation in skin fibroblasts of patients with p.S331 mutations.
Results: In both patients, we recognised an early onset phenotype with prevalent progressive motor neuron disease. Neuropathology showed severe damage to the sensory and autonomic systems. Sphingolipidomic analysis showed the coexistence of neurotoxic deoxy-sphingolipids with an excess of canonical products of the SPT enzyme. l-serine supplementation in patient fibroblasts reduced production of toxic 1-deoxysphingolipids but further increased the overproduction of sphingolipids.
Conclusions: Our findings suggest that p.S331 SPTLC1 variants lead to an overlap phenotype combining features of sensory and motor neuropathies, thus proposing a continuum in the spectrum of SPTLC1-related disorders. l-serine supplementation in these patients may be detrimental.
Keywords: HSAN; S331; SPTLC1; l-serine; motor neuron; sphingolipids.
© 2022 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Conflict of interest statement
Nothing to report.
Figures






Similar articles
-
Serine Palmitoyltransferase (SPT)-related Neurodegenerative and Neurodevelopmental Disorders.J Neuromuscul Dis. 2024;11(4):735-747. doi: 10.3233/JND-240014. J Neuromuscul Dis. 2024. PMID: 38788085 Free PMC article. Review.
-
Recurrent de novo SPTLC2 variant causes childhood-onset amyotrophic lateral sclerosis (ALS) by excess sphingolipid synthesis.J Neurol Neurosurg Psychiatry. 2024 Jan 11;95(2):103-113. doi: 10.1136/jnnp-2023-332132. J Neurol Neurosurg Psychiatry. 2024. PMID: 38041679 Free PMC article.
-
Expanding the spectrum of SPTLC1-related disorders beyond hereditary sensory and autonomic neuropathies: A novel case of the distinct "S331 syndrome".J Peripher Nerv Syst. 2020 Sep;25(3):308-311. doi: 10.1111/jns.12394. Epub 2020 Jun 11. J Peripher Nerv Syst. 2020. PMID: 32470188
-
SPTLC1 variants associated with ALS produce distinct sphingolipid signatures through impaired interaction with ORMDL proteins.J Clin Invest. 2022 Sep 15;132(18):e161908. doi: 10.1172/JCI161908. J Clin Invest. 2022. PMID: 35900868 Free PMC article.
-
Serine Palmitoyltransferase Subunit 3 and Metabolic Diseases.Adv Exp Med Biol. 2022;1372:47-56. doi: 10.1007/978-981-19-0394-6_4. Adv Exp Med Biol. 2022. PMID: 35503173 Review.
Cited by
-
Recurrent de-novo gain-of-function mutation in SPTLC2 confirms dysregulated sphingolipid production to cause juvenile amyotrophic lateral sclerosis.J Neurol Neurosurg Psychiatry. 2024 Feb 14;95(3):201-205. doi: 10.1136/jnnp-2023-332130. J Neurol Neurosurg Psychiatry. 2024. PMID: 38041684 Free PMC article.
-
Serine Palmitoyltransferase (SPT)-related Neurodegenerative and Neurodevelopmental Disorders.J Neuromuscul Dis. 2024;11(4):735-747. doi: 10.3233/JND-240014. J Neuromuscul Dis. 2024. PMID: 38788085 Free PMC article. Review.
-
Human genetic defects of sphingolipid synthesis.J Inherit Metab Dis. 2025 Jan;48(1):e12745. doi: 10.1002/jimd.12745. Epub 2024 May 5. J Inherit Metab Dis. 2025. PMID: 38706107 Free PMC article. Review.
-
Recurrent de novo SPTLC2 variant causes childhood-onset amyotrophic lateral sclerosis (ALS) by excess sphingolipid synthesis.J Neurol Neurosurg Psychiatry. 2024 Jan 11;95(2):103-113. doi: 10.1136/jnnp-2023-332132. J Neurol Neurosurg Psychiatry. 2024. PMID: 38041679 Free PMC article.
-
Spectrum of SPTLC1-related disorders: a novel case of 'Ser331 syndrome' that expand the phenotype of hereditary sensory and autonomic neuropathy type 1A and motor neuron diseases.Neurol Sci. 2023 Jul;44(7):2551-2554. doi: 10.1007/s10072-023-06763-3. Epub 2023 Mar 24. Neurol Sci. 2023. PMID: 36964315
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

